
��Ҫ����գ�
��1����ijҩƷ����ԼΪ32g����������ƽȷ�������������á���ʾ�����̷������룬�á���ʾ������ȡ�£������б���Ŀո��ڣ��á��͡���ʾ��Ӧ����ķ��ϻ�ȡ�¡�
| 50g | 20g | 20g | 10g | 5g |
| ���� | �� |
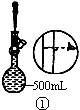
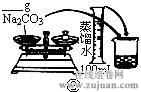
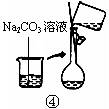
��2������500mL 0.1mol��L -1 Na2CO3��Һ����ͼ��������Ӧ����д������Ϊ ��ʵ��ʱ��ͼ��ʾ�������Ⱥ�˳��Ϊ �����ţ���

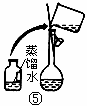

��3��������һ���������ʵ���Ũ����Һʱ���á�ƫ�ߡ�ƫ�͡���Ӱ�족��ʾ���в�����������ҺŨ�ȵ�Ӱ�졣
�ٶ���ʱ���ӣ���������Һ��Ũ�� ��
������ƿϴ�Ӻ�δ�����������Һ��Ũ�� ��
�۶���ҡ�Ⱥ���������Һ��������������Һ��Ũ�� ��
��4������̼���ƹ��壬������ˮ�У����� ����Ӧ�ķ���ʽΪ ��
��1��
| 50g | 20g | 20g | 10g | 5g |
| ���� | �� | ���� | �� | ���� |
��2��5.3 g���ڢܢۢݢ٢ޣ���3���� ƫ�ߣ��� ��Ӱ�죻�� ��Ӱ�죻��4��������Һ��������ð������������ɫ��ζ�����壩������ʽ��Na2CO3+2HCl =2NaCl+CO2��+H2O��
��1��������֪��30gΪ������������Ѽ�20g���롣���ټ�10g���뼴�ɣ���2g�ƶ����롣��2��m(Na2CO3)=0.5L ��0.1mol��L -1��106g��mol -1=5.3g����3���ٸ�������Һ�����С��Ũ��ƫ�ߣ��۶���ҡ�Ⱥ���Һ�������꣬��������Һ������Ũ����Ӱ�졣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����м��ּ�ԭ����У���Ҫ����գ�
�����м��ּ�ԭ����У���Ҫ����գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ϵͳ�����ǣ�
��ϵͳ�����ǣ� �ļ���ʽ��
�ļ���ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com