
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
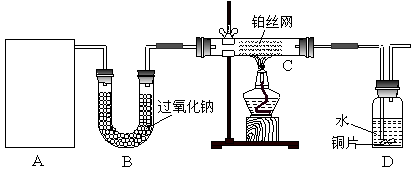
ij��ѧ����С����ʵ�������������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣
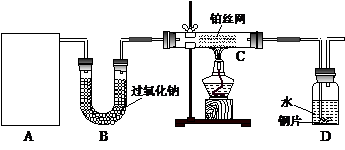
��1��A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������_______________����ѡ���ţ�����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ____________________��ѡ������������ţ��̶�װ��ʡ�ԣ���

��2����װ�ò�����������Ȼ����һ��ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��______________________________________________________________________��
��______________________________________________________________________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B������__________________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ���������2010������ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
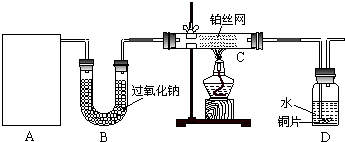
��12�֣�ij��ѧ����С����ʵ�������������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣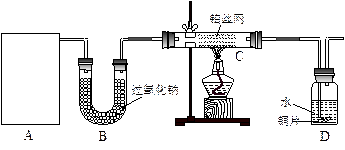
��1��A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������_______________����ѡ���ţ�����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ____________________��ѡ������������ţ��̶�װ��ʡ�ԣ���
��2����װ�ò�����������Ȼ����һ��ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��______________________________________________________________________��
��______________________________________________________________________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B������__________________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ���������2010������ڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣�ij��ѧ����С����ʵ�������������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣
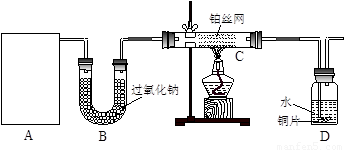
��1��A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������_______________����ѡ���ţ�����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ____________________��ѡ������������ţ��̶�װ��ʡ�ԣ���
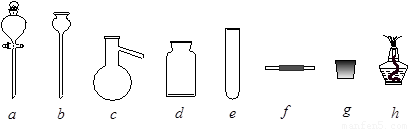
��2����װ�ò�����������Ȼ����һ��ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��______________________________________________________________________��
��______________________________________________________________________��
��3�����ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B������__________________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com