
X”¢Y”¢ZČżÖÖ³£¼ūŌŖĖŲµÄµ„ÖŹ£¬¼×”¢ŅŅŹĒĮ½ÖÖ³£¼ūµÄ»ÆŗĻĪļ£¬Ļą»„¼äÓŠČēĶ¼×Ŗ»Æ¹ŲĻµ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© ČōX»łĢ¬Ō×ÓĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3s2£¬¼×ŹĒÓɵŚ¶žÖÜĘŚĮ½ÖÖŌŖĖŲµÄŌ×Ó¹¹³ÉµÄĘųĢ¬»ÆŗĻĪļ·Ö×Ó£¬ Y»łĢ¬Ō×ӵĹģµĄ±ķŹ¾Ź½ĪŖ £¬¼×µÄµē×ÓŹ½ĪŖ
£Ø2£©ČōXŌ×ӵļŪµē×ÓÅŲ¼ĪŖns£Øn-1£©np£Øn+2£©£¬³£ĪĀĻĀYĪŖŅ×»Ó·¢µÄŅŗĢåĪļÖŹ”¢ŅŅĪŖĪŽÉ«Ņ×ČÜÓŚĖ®µÄĘųĢ唣ŌņZĪŖ £¬×é³ÉYµÄŌŖĖŲµÄ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø3£©ČōX”¢Y¾łĪŖ½šŹōµ„ÖŹ£¬X»łĢ¬Ō×ÓĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3s23p1£¬¼×ĪŖ¾ßÓŠ“ÅŠŌµÄŗŚÉ«¹ĢĢ壬ŌņXÓė¼×·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ £¬Y2+Ąė×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø1£©1s2 2s2
2s2 2p2
2p2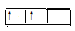 CO2µÄµē×ÓŹ½
CO2µÄµē×ÓŹ½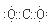
£Ø2£©H2£»[Ar]3d104s24p5
£Ø3£©8Al+3Fe3O4 9Fe+ 4Al2O3 [Ar]3d6
9Fe+ 4Al2O3 [Ar]3d6
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©X»łĢ¬Ō×ÓĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3s2£¬ŌņXµÄŗĖĶāµē×ÓŹżĪŖ12£¬ĖłŅŌXĪŖĆ¾£¬ÓėĆ¾·“Ó¦µÄĘųĢ¬»ÆŗĻĪļÓ¦ĪŖ¶žŃõ»ÆĢ¼£¬Ōņ¼×ĪŖ¶žŃõ»ÆĢ¼£¬YĪŖĢ¼µ„ÖŹ”£ĖłŅŌYµÄ»łĢ¬Ō×ӵĹģµĄ±ķŹ¾Ź½ĪŖ1s2 2s2
2s2 2p2
2p2 ,¶žŃõ»ÆĢ¼µÄµē×ÓŹ½ĪŖ
,¶žŃõ»ÆĢ¼µÄµē×ÓŹ½ĪŖ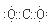
£Ø2£©XŌ×ӵļŪµē×ÓÅŲ¼ĪŖns£Øn-1£©np£Øn+2£©,Ōņn-1=2£¬n=3£¬XµÄ×īĶā²ćµē×ÓÅŲ¼ĪŖ3s23p5£¬XĪŖĀČĘų£»³£ĪĀĻĀYĪŖŅ×»Ó·¢µÄŅŗĢåĪļÖŹ£¬ŌņYĪŖŅŗä壻³£ĪĀĻĀŅŅĪŖĪŽÉ«Ņ×ČÜÓŚĖ®µÄĘųĢ壬ŅŅĪŖĀČ»ÆĒā£¬ZĪŖĒāĘų£»äåŹĒ35ŗÅŌŖĖŲ£¬Ę仳Ģ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ[Ar]3d104s24p5
£Ø3£©X»łĢ¬Ō×ÓĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3s23p1£¬XĪŖĀĮµ„ÖŹ£»¼×ĪŖ¾ßÓŠ“ÅŠŌµÄŗŚÉ«¹ĢĢ壬¼×ĪŖĖÄŃõ»ÆČżĢś£¬XÓė¼×·“Ó¦¼“ĀĮČČ·“Ó¦£¬»Æѧ·½³ĢŹ½ĪŖ8Al+3Fe3O4 9Fe +4Al2O3 £¬YĪŖĢśµ„ÖŹ£¬Fe2+µÄµē×ÓÅŲ¼Ź½ĪŖ[Ar]3d6
9Fe +4Al2O3 £¬YĪŖĢśµ„ÖŹ£¬Fe2+µÄµē×ÓÅŲ¼Ź½ĪŖ[Ar]3d6
æ¼µć£ŗæ¼²éŌŖĖŲĶʶĻ¼°»łĢ¬Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½”¢¹ģµĄ±ķŹ¾Ź½£¬»ÆŗĻĪļµÄµē×ÓŹ½£¬»Æѧ·½³ĢŹ½ŹĒŹéŠ“



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬Õė¶Ō±ķÖŠµÄ¢Ł”«¢āÖŠŌŖĖŲ£¬ÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½»Ų“šŅŌĻĀĪŹĢā£ŗ
| Ö÷×å ÖÜĘŚ | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| ¶ž | | | | | ¢Ł | | ¢Ś | |
| Čż | ¢Ū | ¢Ü | ¢Ż | ¢Ž | | | ¢ß | ¢ą |
| ĖÄ | ¢į | | | | | | ¢ā | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖ£ŗA”¢B”¢C”¢DĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AÓėDµÄŌ×ÓŠņŹżÖ®ŗĶµČÓŚBÓėCµÄŌ×ÓŠņŹżÖ®ŗĶ£¬ÓÉDŌŖĖŲ×é³ÉµÄµ„ÖŹŌŚĶس£×“æöĻĀ³Ź»ĘĀĢÉ«£¬B”¢C”¢DČżÖÖŌŖĖŲĪ»ÓŚĶ¬Ņ»ÖÜĘŚ£¬B”¢C”¢DČżÖÖŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ·Ö±šĪŖX”¢Y”¢Z£¬X”¢Y”¢ZæÉĮ½Į½Ļą»„·“Ӧɜ³ÉŃĪŗĶĖ®£¬ŹŌĶʶĻ²¢ÓĆĻąÓ¦µÄ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā”£
(1)DŌŖĖŲŌ×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ______________________£»
(2)XÓėCŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļæÉŅŌ·¢Éś·“Ó¦£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
____________________________________£»
(3)A”¢B”¢CČżÖÖŌŖĖŲµÄŌ×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖ_________________£»
(4)AÓėDĮ½ŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļÖ®¼äæÉŅŌ·“Ӧɜ³ÉŅ»ÖÖŃĪ£¬øĆŃĪµÄĖ®ČÜŅŗ³Ź________(Ģī”°Ėį”±”°¼ī”±»ņ”°ÖŠ”±)ŠŌ£¬øĆĖ®ČÜŅŗÖŠø÷Ąė×ÓÅضČÓÉŠ”µ½“óµÄĖ³ŠņĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠA”¢B”¢C”¢DĖÄÖÖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬ĘäÖŠAŌŖĖŲŌ×ÓŗĖĶāµē×Ó½öÓŠŅ»ÖÖŌ×Ó¹ģµĄ£¬Ņ²ŹĒÓīÖęÖŠ×ī·įø»µÄŌŖĖŲ£¬BŌŖĖŲŌ×ÓŗĖĶāpµē×ÓŹż±Čsµē×ÓŹżÉŁ1£¬CĪŖ½šŹōŌŖĖŲĒŅŌ×ÓŗĖĶāpµē×ÓŹżŗĶsµē×ÓŹżĻąµČ£¬DŌŖĖŲµÄŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«³äĀś»ņ°ė³äĀś”£
£Ø1£©Š“³öĖÄÖÖŌŖĖŲµÄŌŖĖŲ·ūŗÅ£ŗ
A________£¬B________£¬C________£¬D________”£
£Ø2£©Š“³öC”¢DĮ½ÖÖŌŖĖŲ»łĢ¬Ō×ÓŗĖĶāµē×Ó¹ģµĄ±ķŹ¾Ź½”£
C_______________________________________________________________£¬
D_______________________________________________________________ӣ
£Ø3£©Š“³öB”¢CĮ½ÖÖŌŖĖŲµ„ÖŹŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦µÄ»Æѧ·½³ĢŹ½____________________________________________”£
£Ø4£©Š“³öBŌŖĖŲµ„ÖŹŗĶĒā»ÆĪļµÄµē×ÓŹ½£ŗµ„ÖŹ________£¬Ēā»ÆĪļ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
W”¢X”¢Y”¢ZŹĒĖÄÖÖ³£¼ūµÄ¶ĢÖÜĘŚŌŖĖŲ£¬ĘäŌ×Ó°ė¾¶ĖęŌ×ÓŠņŹż±ä»ÆČēĻĀĶ¼ĖłŹ¾”£ŅŃÖŖWµÄŅ»ÖÖŗĖĖŲµÄÖŹĮæŹżĪŖ18£¬ÖŠ×ÓŹżĪŖ10£»XŗĶNeŌ×ÓµÄŗĖĶāµē×ÓŹżĻą²ī1£»YµÄµ„ÖŹŹĒŅ»ÖÖ³£¼ūµÄ°ėµ¼Ģå²ÄĮĻ£»ZµÄµēøŗŠŌŌŚĶ¬ÖÜĘŚÖ÷×åŌŖĖŲÖŠ×ī“ó”£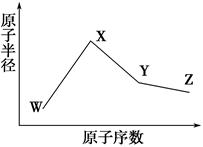
£Ø1£©XĪ»ÓŚŌŖĖŲÖÜĘŚ±ķÖŠµŚ________ÖÜĘŚµŚ________×壻WµÄ»łĢ¬Ō×ÓŗĖĶāÓŠ________øöĪ“³É¶Ōµē×Ó”£
£Ø2£©ZµÄĘųĢ¬Ēā»ÆĪļŗĶäå»ÆĒāĻą±Č£¬½ĻĪČ¶ØµÄŹĒ________________£ØŠ“»ÆѧŹ½£©”£
£Ø3£©YÓėZŠĪ³ÉµÄ»ÆŗĻĪļŗĶ×ćĮæĖ®·“Ó¦£¬Éś³ÉŅ»ÖÖČõĖįŗĶŅ»ÖÖĒæĖį£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ¶ĢÖÜĘŚÖŠŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ5ÖÖÖ÷×åŌŖĖŲ£¬ĘäÖŠŌŖĖŲAµÄµ„ÖŹŌŚ³£ĪĀĻĀ³ŹĘųĢ¬£¬ŌŖĖŲBµÄŌ×Ó×īĶā²ćµē×ÓŹżŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬ŌŖĖŲCŌŚĶ¬ÖÜĘŚµÄÖ÷×åŌŖĖŲÖŠŌ×Ó°ė¾¶×ī“ó£¬ŌŖĖŲDµÄŗĻ½šŹĒČÕ³£Éś»īÖŠ³£ÓĆµÄ½šŹō²ÄĮĻ”£³£ĪĀ³£Ń¹ĻĀ£¬Eµ„ÖŹŹĒµ»ĘÉ«¹ĢĢ壬³£ŌŚ»šÉ½æŚø½½ü³Į»ż”£
£Ø1£©DŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ____________,CŗĶEĄė×Ó°ė¾¶“óŠ”±Č½Ļ______________”£
£Ø2£©A2EµÄČ¼ÉÕČȦ¤H £½ £a kJ/mol£¬Š“³öA2EČ¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ_______________”£
£Ø3£©CAµÄµē×ÓŹ½ĪŖ ___________£»ABŠĪ³ÉµÄ¾§ĢåČŪµć_______”££ØĢī”°“óÓŚ”±”°Š”ÓŚ”±»ņ”°ĪŽ·ØÅŠ¶Ļ”±£©CEŠĪ³ÉµÄ¾§ĢåČŪµć”£
£Ø4£©¼×”¢ŅŅ”¢±ū·Ö±šŹĒB”¢D”¢EČżÖÖŌŖĖŲ×īøß¼Ūŗ¬ŃõĖįµÄÄĘŃĪ£¬¼×”¢ŅŅ¶¼ÄÜÓė±ū·¢Éś·“Ó¦£¬ĒŅ±ūÓĆĮæ²»Ķ¬£¬·“Ó¦µÄ²śĪļ²»Ķ¬”£Š“³öĻņ±ūČÜŅŗÖŠ»ŗĀżµĪ¼Ó¹żĮæµÄŅŅČÜŅŗ¹ż³ĢÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
±źŗÅĪŖ¢Ł”«¢āµÄŌŖĖŲ£¬ŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĻĀ£ŗ
| Ö÷×å ÖÜĘŚ”” | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0×å |
| 1 | ¢Ł | | | | | | | ¢Ś |
| 2 | | | | ¢Ū | ¢Ü | ¢Ż | ¢Ž | |
| 3 | ¢ß | ¢ą | | | | ¢į | ¢ā | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶ĢÖÜĘŚŌŖĖŲR”¢Q”¢M”¢TŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĻĀ±ķ£¬ŅŃÖŖRŌ×Ó×īĶā²ćµē×ÓŹżÓė“ĪĶā²ćµē×ÓŹżÖ®±ČĪŖ2£ŗ1”£
£Ø1£©ČĖµÄŗ¹ŅŗÖŠĢØÓŠTµÄ¼ņµ„Ąė×Ó£¬ĘäĄė×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ_________________£»ŌŖĖŲMŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ___________________”£
£Ø2£©RµÄ×īøß¼ŪŃõ»ÆĪļĖłŗ¬µÄ»Æѧ¼üĄąŠĶŹĒ__________¼ü(Ń”Ģī”°Ąė×Ó”±»ņ”°¹²¼Ū”±)”£
£Ø3£©¼ÓČČŹ±£¬QµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄÅØČÜŅŗÓėµ„ÖŹR·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________(ÓĆ¾ßĢåµÄ»ÆѧŹ½±ķŹ¾)”£
£Ø4£©ŌŚŅ»¶ØĢõ¼žĻĀ¼×”¢ŅŅ”¢±ūÓŠČēĻĀ×Ŗ»Æ£ŗ¼×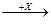 ŅŅ
ŅŅ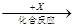 ±ū£¬ČōĘäÖŠ¼×ŹĒµ„ÖŹ£¬ŅŅ”¢±ūĪŖ»ÆŗĻĪļ£¬xŹĒ¾ßÓŠŃõ»ÆŠŌµÄĪŽÉ«ĘųĢåµ„ÖŹ£¬Ōņ¼×µÄ»Æѧ×é³É²»æÉÄÜŹĒ________(Ń”ĢīŠņŗÅ£¬ĻĀĶ¬)”£
±ū£¬ČōĘäÖŠ¼×ŹĒµ„ÖŹ£¬ŅŅ”¢±ūĪŖ»ÆŗĻĪļ£¬xŹĒ¾ßÓŠŃõ»ÆŠŌµÄĪŽÉ«ĘųĢåµ„ÖŹ£¬Ōņ¼×µÄ»Æѧ×é³É²»æÉÄÜŹĒ________(Ń”ĢīŠņŗÅ£¬ĻĀĶ¬)”£
¢ŁR ¢ŚQ2 ¢ŪM ¢ÜT2
£Ø5£©¹¤ŅµÉĻ£¬³£ĄūÓĆ”£ROÓėMO2·“Ӧɜ³É¹ĢĢ¬Mµ„ÖŹŗĶRO2£¬“Ó¶ųĻū³żÕāĮ½ÖÖĘųĢå¶Ō“óĘųµÄĪŪČ¾”£
ŅŃÖŖ£ŗ2RO(g)+O2(g)£½2RO2(g) ”÷H£½-akJ”¤mol-l
M(s)+O2(g)£½MO2(g) ”÷H£½-bkJ”¤mol-l
Ōņ·“Ó¦2RO(g)+MO2(g)£½2RO2(g)+M(s) ”÷H£½___________”£
£Ø6£©ŌŖĖŲTµÄŗ¬ŃõĖįHTO¾ßÓŠĘư׊Ō”£Ķł20mL 0.0lmol”¤L-lµÄHTOČÜŅŗÖŠÖšµĪ¼ÓČėŅ»¶ØÅØ¶ČµÄÉÕ¼īČÜŅŗ£¬²āµĆ»ģŗĻČÜŅŗµÄĪĀ¶Č±ä»ÆČēĻĀĶ¼ĖłŹ¾”£¾Ż“ĖÅŠ¶Ļ£ŗøĆÉÕ¼īČÜŅŗµÄÅضČĪŖ______________mol”¤L-l£»ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ_______________”£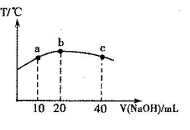
¢ŁHTOµÄµēĄė³£Źż£ŗbµć£¾aµć
¢ŚÓÉĖ®µēĄė³öµÄc(OH”Ŗ)£ŗbµć£¼cµć
¢Ū“Óaµćµ½bµć£¬»ģŗĻČÜŅŗÖŠæÉÄÜ“ęŌŚ£ŗc(TO”Ŗ)= c(Na+)
¢Ü“Óbµćµ½cµć£¬»ģŗĻČÜŅŗÖŠŅ»Ö±“ęŌŚ£ŗc(Na£«)£¾c(TO£)£¾c(OH£)£¾c(H£«)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ŹĒŌŖĖŲÖÜĘŚ±ķÖŠ½ŲČ”µÄŅ»øöʬ¶Ī,ĘäÖŠX”¢Y”¢Z”¢W¾łŹōÓŚ¶ĢÖÜĘŚŌŖĖŲ”£Ēė»Ų“šĻĀĮŠĪŹĢā:
(1)ČōXµ„ÖŹĪŖæÕĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»,ŌņWŌ×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ”””””””””£
(2)ČōY”¢W”¢ZµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŅĄ“ĪĪŖŅ»ŌŖĖį”¢¶žŌŖĖį”¢ČżŌŖĖį,ÕāČżÖÖĖįµÄÕżŃĪµÄŗ¬ŃõĖįøłĄė×ÓÖŠ,ÓŠĮ½Öֵĵē×ÓŹżĻąµČ,ŌņÕāĮ½ÖÖĄė×ӵķūŗÅŹĒ”””””””””¢”””””””””£
(3)ČōNaÓėYŗĶZµÄµ„ÖŹ·Ö±š·“Ó¦æÉŅŌÉś²śÄ¦¶ūÖŹĮæĻąĶ¬µÄĮ½ÖÖ»ÆŗĻĪļ,ĘäÖŠNaÓėYŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ””””””””””””””,Ėłŗ¬»Æѧ¼üµÄĄąŠĶĪŖ””
(4)YŌŖĖŲŌŚÖÜĘŚ±ķÖŠæÉÄÜ“¦ÓŚµŚ””””””””””””””””ׯŠŠ(Š“³öĖłÓŠæÉÄÜׯŠŠŹż)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com