
�������֣���1��30%��H2SO4��50%��H2SO4��������Ϻ�������Һ����������________(����ڡ�����С�ڡ����ڡ�)40%������������ϣ���Ϻ���Һ����������________(����ڡ�����С�ڡ����ڡ�)40%��
��2����֪98%��ŨH2SO4�����ʵ���Ũ��Ϊ18.4 mol��L��1�����ж�49%��H2SO4�����ʵ���Ũ��________(����ڡ�����С�ڡ����ڡ�)9.2 mol��L��1��
��3����һ���¶Ⱥ�ѹǿ�£�1���X2(g)��3���Y2(g)��������2���Z(g)����Z����Ļ�ѧʽ��________ ��
��4���ڱ�״���£�CO��CO2�Ļ����������Ϊ36 g�����Ϊ22.4 L����CO��ռ�������__________ L��������________ g��
��1������ ���� ��2��С�ڣ�3��XY3 ��4��11.2 14
��������
�����������1����ԭ��������Ϊm��30%��H2SO4����Ϊ����0.4m��50%��H2SO4���ʵ�����Ϊ0.5m����Ϻ���������仯���������䣬����w��H2SO4��=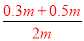 ��100%=40%������������ϣ�������ֱ�ΪVml��30%��H2SO4��Һ�ܶ�Ϊ��1��50%��H2SO4��Һ�ܶ�Ϊ��2�����Ϻ���Һ����������Ϊw��H2SO4��=
��100%=40%������������ϣ�������ֱ�ΪVml��30%��H2SO4��Һ�ܶ�Ϊ��1��50%��H2SO4��Һ�ܶ�Ϊ��2�����Ϻ���Һ����������Ϊw��H2SO4��=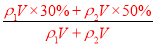 ��100%���������Ũ��Խ���ܶ�Խ�����1����2����w��H2SO4����40%����2��������c=
��100%���������Ũ��Խ���ܶ�Խ�����1����2����w��H2SO4����40%����2��������c=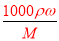 ���㣬��98%��ŨH2SO4���ܶ�Ϊx��49%��H2SO4��Һ���ܶ�Ϊy�����ʵ���Ũ��Ϊc����
���㣬��98%��ŨH2SO4���ܶ�Ϊx��49%��H2SO4��Һ���ܶ�Ϊy�����ʵ���Ũ��Ϊc���� =18.4��
=18.4�� =c�����ԣ�
=c�����ԣ�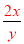 =
=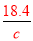 ���������Ũ��Խ���ܶ�Խ����x��y����c��9.2mol/L����3�����ݰ����ӵ����ɿ�֪��1molX2��3molY2��������2mol�����廯������Ը���ԭ���غ㼴�����غ㶨�ɿ�֪���û�����Ļ�ѧʽ��XY3����4����CO�����ʵ���ΪXmol��CO2�����ʵ���ΪYmol����28X+44Y=36��X+Y=1�����X=Y=0.5����CO�����=0.5mol��22.4L/mol=11.2L ,����Ϊ=0.5mol��28g/mol=14g��
���������Ũ��Խ���ܶ�Խ����x��y����c��9.2mol/L����3�����ݰ����ӵ����ɿ�֪��1molX2��3molY2��������2mol�����廯������Ը���ԭ���غ㼴�����غ㶨�ɿ�֪���û�����Ļ�ѧʽ��XY3����4����CO�����ʵ���ΪXmol��CO2�����ʵ���ΪYmol����28X+44Y=36��X+Y=1�����X=Y=0.5����CO�����=0.5mol��22.4L/mol=11.2L ,����Ϊ=0.5mol��28g/mol=14g��
���㣺��ҺŨ�ȵļ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015������ʡ�������ɽ�ظ�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����100 mL 1.0 mol��LNa2CO3��Һ�����в�����ȷ����
A����ȡ10.6 g��ˮ̼���ƣ�����100 mL����ƿ�У���ˮ�ܽ⡢����
B����ȡ10.6 g��ˮ̼���ƣ�����100 mL����ˮ�����衢�ܽ�
C��ת��Na2CO3��Һʱ��δ�ò�����������ֱ�ӵ�������ƿ��
D�����ݺ�����ƿ����������ת��ҡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������и������ڵ�һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A��Ũ���ᱣ���ڲ���������ɫ�Լ�ƿ��
B���Ѿ���ɶ��ݵ�500 mL 1.0 mol��L-1 ��NaOH��Һ��ijͬѧ����������ƿ����Һ������ֻ����������
C�����ܿڵ�ȼCOʱ��Ҫ�鴿��H2���Ȼ�ԭCuOʱҲ��Ҫ�鴿
D����������ˮ�Ĺ����У�һ���з��Ȼ�������������ܽ�IJ�����������Ͳ�н���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������0.1mol/L��NH4CN��Һ��pH����9.32������˵���������
A��������Һ��ʹ�����Լ����ɫ��ʯ����Һ����
B�������£�HCN�DZ�NH3��H2O�����ĵ����
C��������Һ��NH4+��ˮ��̶ȴ���CN-��ˮ��̶�
D�������£�0.1mol/LNaCN��Һ�У�CN-��ˮ��̶�С��������Һ��CN-��ˮ��̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������һ���л��ᣬ�ֽ����ᡣ���ӡ��䡢���ϵ����涣ҧ��ʱ�����������������ᣬʹƤ�����ס�������������ʹ��Ҫ��������֢״�����ڶ�ҧ��ͿĨ���������е�
��֪��ͥ��һЩ�������ʵ�pH��ʳ��-4������-9��ʳ��ˮ-7������ˮ-10�����Һ-13
A�����Һ B��ʳ��ˮ C����������ˮ D��ʳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���ܴﵽĿ�ĵ���
A����ŨFeCl3��Һ��NaOH��Һ����Ʊ�Fe(OH)3����
B������������HCl��CO2ͨ�뱥��NaHCO3��Һ�г�ȥHCl
C����ʪ���pH��ֽ�ⶨ��Ũ�ȵ�Na2CO3��Na2SO3 ��Һ��pH��С
D����AgNO3��Һ����Na2SO4��Һ��NaCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ƫ������(C2H8N2)��һ�ָ���ȼ�ϣ�ȼ�ղ���������������Ϊ�������ػ�����ƶ�����������������ȷ����
A��ƫ�����µ�Ħ������Ϊ60 g��mol��1
B��6.02��1023��ƫ�����·��ӵ�����Ϊ60
C��1 molƫ�����µ�����Ϊ60 g��mol��1
D��6 gƫ�����º���NA��ƫ�����·���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ������ѧ��10���¿��Ծ��������棩 ���ͣ�ѡ����
30mlCO2��NO�������ͨ��������Na2O2���岢�ҳ�ַ�Ӧ��,���������Ϊ20ml����ԭ���������CO2���Ϊ��ͬ��ͬѹ�£� ( )
A��30mL B��20mL C��10mL D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��У������ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
NA���������ӵ�����,����˵����ȷ����( )
A��0.5 molAl���������ᷴӦת�Ƶ�����Ϊ1NA
B����״���£�11.2L SO3�����ķ�����Ϊ0.5NA
C��0.1molCH4�����ĵ�����Ϊ1NA
D��46gNO2��N2O4�Ļ���ﺬ�еķ�����Ϊ1NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com