
A��B��C��һ�������½������л�ѧ��Ӧ����������µ�ת����ϵ�ش���
�����⣬��֪A��B��C�о�����ͬһ��Ԫ�ء�
1��DΪ�ǽ������ʣ���A��B��C֮���������ת����ϵ��
![]()
����AΪ�������ʣ� C��ˮ��Ӧ�ܹ�����D��д��C�ĵ���ʽ
����AΪ��̬�����C�ķ���ʽ������ �����������֣�
����AΪ���ĺ��������A�����к���2��̼ԭ�ӡ������£�23g A��ȫȼ�շų�683.5kJ��������д���ܱ�ʾAȼ���ȵ��Ȼ�ѧ����ʽ
����DΪ�������ʣ������Ϸ�Ӧ��Ϊ������ԭ��Ӧ����д������B�������ӵ�һ�ַ��� ��
����DΪ�BΪ��ɫ�����������Ϸ�Ӧ��Ϊ��������ԭ��Ӧ��д��B��D��Ӧ�����ӷ���ʽ ��
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
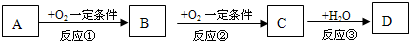
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Ũ���� |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
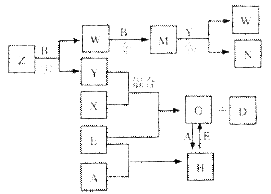 A��B��C��D��E����ѧ��ѧ���������ֵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ��̬��EΪ����������D�ֱܷ��A��B��C��һ�������»��ϣ����ɶ�Ӧ�Ļ�����X��Y��Z�����г����£�YΪҺ�壬X��ZΪ���塣�йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ����
A��B��C��D��E����ѧ��ѧ���������ֵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ��̬��EΪ����������D�ֱܷ��A��B��C��һ�������»��ϣ����ɶ�Ӧ�Ļ�����X��Y��Z�����г����£�YΪҺ�壬X��ZΪ���塣�йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ����
��1��������Z�Ŀռ乹��Ϊ ������A��Ԫ�ص����������ˮ����Ļ�ѧʽΪ ��ʵ��������N�ķ����� ��
��2��д����Ӧ�ڵĻ�ѧ��Ӧ����ʽ ��G��H�����ӷ���ʽ ��
��3�������£�D��A��Ӧ����1 mol Xʱ����92.3kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
��
��4����������E����N��ϡ��Һ�У�����Ӧ������ת�Ƶ��ӵ���ĿΪ3.01��1023����μӷ�Ӧ��E������Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��������ѧ�����ĵ��ʣ���֪A��B��C��D�ڳ����¾�Ϊ��̬������D�ֱܷ��A��B��C��һ���������������ϣ����ɻ�����X��Y��Z��A��B��C������ֱ�ӻ��ϣ��йص�ת����ϵ����ͼ��ʾ����Ӧ����������ȥ���ش��������⣺
��Z���ӵĿռ乹��Ϊ
�Ʒ�Ӧ���б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ ��
��Z��W�ڴ��������£���Ӧ����C��Y������һ������ʵ������ķ�Ӧ����������W�Ի�������Ⱦ���÷�Ӧ�ķ���ʽΪ ��
�Ƚ�������E�ӵ�N��ϡ��Һ�У���������ת���ĵ�����ĿΪ3.02��1023������μӷ�Ӧ��E�����ʵ���Ϊ mol��
�ɽ�Z��N��Ӧ��IJ�������ˮ�У�����Һ��pHֵ���<����>���� 7����ԭ���ǣ������ӷ���ʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com