
·ÖĪö KW=C£ØH+£©•C£ØOH-£©=10-14£¬KWŹĒÖ»ÓėĪĀ¶ČÓŠ¹ŲµÄ³£Źż£¬0.1mol/L µÄHAČÜŅŗÖŠc£ØH+£©=10-10£¬ČÜŅŗÖŠHAÖŠµÄĒāĄė×ÓĪ“Č«²æµēĄė£¬ĖµĆ÷HAĪŖČõĖį£¬0.01mol/LµÄBOHČÜŅŗpH=12£®ČÜŅŗÖŠĒāĄė×ÓÅضČ=0.01mol/mol£¬ĖµĆ÷BOHĪŖĒæ¼ī£¬
£Ø1£©ĶźČ«µēĄėµÄµē½āÖŹĪŖĒæµē½āÖŹ£¬²æ·ÖµēĄėµÄµē½āÖŹĪŖČõµē½āÖŹ£»
£Ø2£©HAĪŖČõĖį“ęŌŚµēĄėĘ½ŗā£»
£Ø3£©ŌŚ¼ÓĖ®Ļ”ŹĶHAµÄ¹ż³ĢÖŠ£¬“Ł½ųHAµēĄėĘ½ŗāÕżĻņ½ųŠŠ£¬Ę½ŗāדĢ¬ĻĀµÄĪ¢Į£ÅØ¶Č¼õŠ”£¬ČÜŅŗÖŠ“ęŌŚĄė×Ó»ż³£Źż£¬ĪĀ¶Č²»±ä£¬Ąė×Ó»ż³£Źż²»±ä£»
£Ø4£©³£ĪĀĻĀpH=12µÄBOHČÜŅŗÖŠc£ØOH-£©=$\frac{1{0}^{-14}}{1{0}^{-12}}$mol/L=0.01mol/L£¬ĮņĖįĒāÄĘŹĒĒæĖįĖįŹ½ŃĪ£¬ĘäĶźČ«µēĄėÉś³ÉÄĘĄė×Ó”¢ĒāĄė×ÓŗĶĮņĖįøłĄė×Ó£¬Ļąµ±ÓŚĒæĖįŠŌČÜŅŗ£¬ĮņĖįĒāÄĘČÜŅŗÖŠc£ØH+£©=0.001mol/L£¬»ģŗĻČÜŅŗpH=11£¾7£¬»ģŗĻČÜŅŗÖŠ$\frac{1{0}^{-14}}{1{0}^{-11}}$mol/L=0.001mol/L£¬Ōņ»ģŗĻČÜŅŗ³Ź¼īŠŌ£¬»ģŗĻČÜŅŗÖŠc£ØOH-£©=$\frac{0.01mol/L”Į0.1L-0.001mol/L”ĮVL}{£Ø0.1+V£©L}$=0.001mol/L£¬¾Ż“Ė¼ĘĖćVÖµ£®
½ā“š ½ā£ŗKW=C£ØH+£©•C£ØOH-£©=10-14£¬KWŹĒÖ»ÓėĪĀ¶ČÓŠ¹ŲµÄ³£Źż£¬0.1mol/L µÄHAČÜŅŗÖŠc£ØH+£©=10-10£¬ČÜŅŗÖŠHAÖŠµÄĒāĄė×ÓĪ“Č«²æµēĄė£¬ĖµĆ÷HAĪŖČõĖį£¬0.01mol/LµÄBOHČÜŅŗpH=12£®ČÜŅŗÖŠĒāĄė×ÓÅضČ=0.01mol/mol£¬ĖµĆ÷BOHĪŖĒæ¼ī£¬
£Ø1£©ÉĻŹö·ÖĪöæÉÖŖ£¬HAĪŖČõĖįĪŖČõµē½āÖŹ£¬BOHŹĒĒæ¼īŹōÓŚĒæµē½āÖŹ£¬¹Ź“š°øĪŖ£ŗČõµē½āÖŹ£»Ēæµē½āÖŹ£»
£Ø2£©HAĪŖČõĖį“ęŌŚµēĄėĘ½ŗā£¬µēĄė·½³ĢŹ½ĪŖ£ŗHA?H++A-£¬¹Ź“š°øĪŖ£ŗHA?H++A-£»
£Ø3£©ŌŚ¼ÓĖ®Ļ”ŹĶHAµÄ¹ż³ĢÖŠ£¬“Ł½ųHAµēĄėĘ½ŗāÕżĻņ½ųŠŠ£¬Ę½ŗāדĢ¬ĻĀµÄĪ¢Į£ÅØ¶Č¼õŠ”£¬ČÜŅŗÖŠ“ęŌŚĄė×Ó»ż³£Źż£¬ĪĀ¶Č²»±ä£¬Ąė×Ó»ż³£Źż²»±ä£¬
A£®¼ÓĖ®Ļ”ŹĶ“Ł½ųµēĄė£¬$\frac{c£Ø{H}^{+}£©}{c£ØHA£©}$Ö®±ČæÉŅŌ±Č½ĻĪļÖŹµÄĮæÖ®±Č£¬Ōņ±ČÖµŌö“󣬹ŹAÕżČ·£»
B£®¼ÓĖ®Ļ”ŹĶ“Ł½ųµēĄė£¬$\frac{c£ØHA£©}{c£Ø{A}^{-}£©}$Ö®±ČæÉŅŌ±Č½ĻĪļÖŹµÄĮæÖ®±Č£¬±ČÖµ¼õŠ”£¬¹ŹB“ķĪó£»
C£®ĪĀ¶Č²»±ä£¬ČÜŅŗÖŠĄė×Ó»ż³£Źż²»±ä£¬c£ØH+£©Óėc£ØOH-£©µÄ³Ė»ż²»±ä£¬¹ŹC“ķĪó£»
D£®¼ÓĖ®Ļ”ŹĶ£¬ČÜŅŗÖŠĒāĄė×ÓÅØ¶Č¼õŠ”£¬c£ØH+£©Óėc£ØOH-£©µÄ³Ė»ż²»±ä£¬c£ØOH-£©Ōö“󣬹ŹDÕżČ·£»
¹Ź“š°øĪŖ£ŗAD£»
£Ø4£©³£ĪĀĻĀpH=12µÄBOHČÜŅŗÖŠc£ØOH-£©=$\frac{1{0}^{-14}}{1{0}^{-12}}$mol/L=0.01mol/L£¬ĮņĖįĒāÄĘŹĒĒæĖįĖįŹ½ŃĪ£¬ĘäĶźČ«µēĄėÉś³ÉÄĘĄė×Ó”¢ĒāĄė×ÓŗĶĮņĖįøłĄė×Ó£¬Ļąµ±ÓŚĒæĖįŠŌČÜŅŗ£¬ĮņĖįĒāÄĘČÜŅŗÖŠc£ØH+£©=0.01mol/L£¬»ģŗĻČÜŅŗpH=11£¾7£¬»ģŗĻČÜŅŗÖŠ$\frac{1{0}^{-14}}{1{0}^{-11}}$mol/L=0.001mol/L£¬Ōņ»ģŗĻČÜŅŗ³Ź¼īŠŌ£¬»ģŗĻČÜŅŗÖŠc£ØOH-£©=$\frac{0.01mol/L”Į0.1L-0.001mol/L”ĮVL}{£Ø0.1+V£©L}$=0.001mol/L£¬x=450mL£¬
¹Ź“š°øĪŖ£ŗ450mL£®
µćĘĄ ±¾Ģāæ¼²éČõµē½āÖŹµÄµēĄė”¢ŃĪĄąĖ®½ā”¢Ėį¼ī»ģŗĻČÜŅŗ¶ØŠŌÅŠ¶ĻµČÖŖŹ¶µć£¬ĪŖøßĘµæ¼µć£¬ÕżČ·ÅŠ¶Ļµē½āÖŹĒæČõŹĒ½ā±¾Ģā¹Ų¼ü£¬ÄѵćŹĒ»ģŗĻČÜŅŗÓŠ¹Ų¼ĘĖć£¬×¢Ņā£ŗĄė×Ó»ż³£ŹżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬ÓėČÜŅŗĖį¼īŠŌ¼°ÅضČĪŽ¹Ų£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓƱ„ŗĶNa2CO3ČÜŅŗ³żČ„CO2ÖŠ»ģÓŠµÄHClĘųĢå | |
| B£® | ÓĆĮæĶ²ĮæČ”20.83mL12mol/LµÄÅØĮņĖįÅäÖĘ250mL1mol/LĻ”ĮņĖį | |
| C£® | ÓĆÅØĮņĖįøÉŌļCO2”¢SO2”¢HIµČĖįŠŌĘųĢå | |
| D£® | ÓĆĻ”HNO3Äܼų±šMg”¢Na2CO3”¢NaAlO2”¢Na2SiO3ĖÄÖÖ¹ĢĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃéŠņŗÅ | ·“Ó¦ĪĀ¶Č/”ę | Na2S2O3ČÜŅŗ | Ļ”H2SO4 | H2O | ||
| V/mL | c/£Ømol/L£© | V/mL | c/£Ømol/L£© | V/mL | ||
| ¢Ł | 20 | 10.0 | 0.10 | 10.0 | 0.50 | 0 |
| ¢Ś | 40 | V1 | 0.10 | V2 | 0.50 | V3 |
| ¢Ū | 20 | V4 | 0.10 | 4.0 | 0.50 | V5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCl”¢HBr”¢HIµÄČČĪČ¶ØŠŌŅĄ“ĪŌöĒæ | B£® | KOHµÄ¼īŠŌ±ČNaOHµÄ¼īŠŌĒæ | ||
| C£® | HBrO4ĖįŠŌ±ČHClO4Ēæ | D£® | NaµÄ½šŹōŠŌ±ČAlµÄČõ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
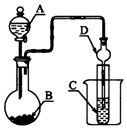
| A£® | ČōAĪŖÅØĮņĖį£¬BĪŖNa2SO3¹ĢĢ壬CÖŠŹ¢ŹÆČļČÜŅŗ£¬ŌņCÖŠČÜŅŗĻȱäŗģŗóĶŹÉ« | |
| B£® | ČōAĪŖ“×ĖįČÜŅŗ£¬BĪŖ±“æĒ£¬CÖŠŹ¢¹żĮæ³ĪĒåŹÆ»ŅĖ®£¬ŌņCÖŠČÜŅŗ±ä»ė×Ē | |
| C£® | ČōAĪŖÅØŃĪĖį£¬BĪŖMnO2£¬CÖŠŹ¢Ę·ŗģČÜŅŗ£¬ŌņCÖŠČÜŅŗ²»ĶŹÉ« | |
| D£® | ČōAĪŖÅØ°±Ė®£¬BĪŖÉśŹÆ»Ņ£¬CÖŠŹ¢AlCl3ČÜŅŗ£¬ŌņCÖŠ²śÉś°×É«³Įµķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÄÜŹ¹¹ć·ŗpHŹŌÖ½ĻŌŗģÉ«µÄČÜŅŗ£ŗK+”¢Ba2+”¢Cl-”¢Br- | |
| B£® | ŗ¬ÓŠ“óĮæAl3+µÄČÜŅŗ£ŗNa+”¢Cl-”¢HCO3-”¢SO42- | |
| C£® | ÄÜŹ¹µķ·Ūµā»Æ¼ŲŹŌÖ½ĻŌĄ¶É«µÄČÜŅŗ£ŗK+”¢SO42-”¢S2-”¢SO32- | |
| D£® | ³£ĪĀĻĀ$\frac{c£Ø{H}^{+}£©}{c£ØO{H}^{-}£©}$=1012µÄČÜŅŗ£ŗFe2+”¢Mg2+”¢NO3-”¢Cl- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com