
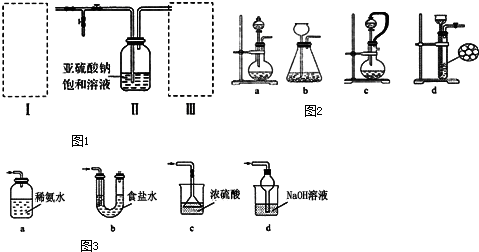
���� ��1���������ƹ����Ũ�����Ʊ������������壬��Ӧ���������ơ�����������ˮ�����Ƽ���������ٶȣ����Կ��Ʒ�Ӧ�ٶȣ�
��2����֪����H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8KJ/mol
��C��s��+$\frac{1}{2}$O2��g���TCO��g����H=-110.5KJ/mol
���ݸ�˹���ɣ���-�ٿɵã�C��s��+H2O��g��=CO��g��+H2��g��
��3����������������������������������Ʒ�Ӧ�����������Ա�̼��ǿ������̼���Ʒ�Ӧ��������CaCl2��NaHSO3��Ӧ��
��4�����뾧������Һ��ȡ���˷�����
Ӧѡ��500mL����ƿ������n=cV���㽹�������Ƶ����ʵ������ٸ���m=nM���㽹�������Ƶ�������������Һ��Ҫ�õ��������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
��5������β���ж�������ֹ��Ⱦ�����������ü�Һ���գ���ֹ��������ֹ����������
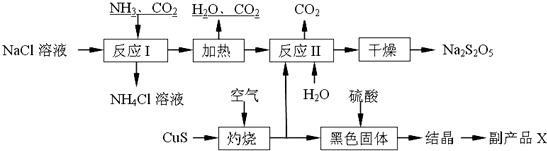
��6���Ȼ�����Һ����ͨ�백������ͨ�������̼����̼�����ƾ��壬���˷��룬̼�����Ƽ��ȷֽ�õ�̼���ƣ�CuS���յõ�����������CuO����������ˮ��̼����ϵ�з�Ӧ�õ�Na2S2O5��CuO�������ܽ�õ�����ͭ��Һ����������Ũ������ȴ�ᾧ�����˵Ȳ����õ�CuSO4•5H2O��
��ʵ���������Ȼ�������������ڼ����������Ʊ�������
�ڡ����ա�ʱCuS��������Ӧ��������ͭ���������
��Na2S2O5��ϡ���ᷴӦ�ų�SO2����Ӧ��SԪ�ػ��ϼ�û�б仯��������ˮ��
�ܸ���ƷX�Ļ�ѧʽ��CuSO4•5H2O���ɹ������̿�֪����ѭ�����õ������ǣ�CO2��H2O��
���ɻ�ѧʽNa2S2O5��Ӧ����ԭ����Na��Sԭ����Ŀ֮��Ϊ1��1��
��� �⣺��1���������ƹ����Ũ�����Ʊ������������壬��Ӧ���������ơ�����������ˮ����Ӧ����ʽΪ��Na2SO3+H2SO4�TNa2SO4+SO2��+H2O�����Ƽ���������ٶȣ����Կ��Ʒ�Ӧ�ٶȣ������÷�Һ©���������ᣬ���ڿ��Ƶμ������ٶȣ�
�ʴ�Ϊ��Na2SO3+H2SO4�TNa2SO4+SO2��+H2O��ac��
��2����֪����H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H1=-241.8kJ•mol-1
��C��s��+$\frac{1}{2}$O2��g���TCO��g����H2=-110.5kJ•mol-1
���ݸ�˹���ɣ���-�ٿɵã�C��s��+H2O��g��=CO��g��+H2��g����H=+131.3kJ•mol-1
�ʴ�Ϊ��C��s��+H2O��g��=CO��g��+H2��g����H=+131.3kJ•mol-1 ��
��3����������������������������������Ʒ�Ӧ�����������Ա�̼��ǿ������̼���Ʒ�Ӧ��������CaCl2��NaHSO3��Ӧ����ѡ��ab��
��4�����뾧������Һ��ȡ���˷�����Ӧѡ��500mL����ƿ�����������Ƶ����ʵ���Ϊ0.5L��0.1mol��L=0.05mol������Ҫ���������Ƶ�����Ϊ0.05mol��190g/mol=9.5g��������Һ��Ҫ�õ��������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ�����ˣ�9.5g��500mL����ƿ����ͷ�ιܣ�
��5������β���ж�������ֹ��Ⱦ������
a������װ�ô�����ȫ�ܱջ�����װ������ѹ���ᷢ��ը��Σ�գ���a����
b��װ�ô����ܱ�״̬��������������������Һѹ��U�ܣ����Ȼ�����Һ���ն���������ȫ����b����
c��Ũ��������ն�������c����
d���������ƿ������ն�������װ�ÿ��Է�ֹ��������d��ȷ��
��ѡ��d��
��6���Ȼ�����Һ����ͨ�백������ͨ�������̼����̼�����ƾ��壬���˷��룬̼�����Ƽ��ȷֽ�õ�̼���ƣ�CuS���յõ�����������CuO����������ˮ��̼����ϵ�з�Ӧ�õ�Na2S2O5��CuO�������ܽ�õ�����ͭ��Һ����������Ũ������ȴ�ᾧ�����˵Ȳ����õ�CuSO4•5H2O��
��ʵ������ȡ�����Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl $\frac{\underline{\;\;��\;\;}}{\;}$ CaCl2+2H2O+2NH3����
�ʴ�Ϊ��Ca��OH��2+2NH4Cl $\frac{\underline{\;\;��\;\;}}{\;}$ CaCl2+2H2O+2NH3����
�ڡ����ա�ʱ������Ӧ�Ļ�ѧ����ʽ��2CuS+3O2$\frac{\underline{\;����\;}}{\;}$2CuO+2SO2 ��
�ʴ�Ϊ��2CuS+3O2$\frac{\underline{\;����\;}}{\;}$2CuO+2SO2 ��
����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪ��S2O52-+2H+=2SO2��+H2O��
�ʴ�Ϊ��S2O52-+2H+=2SO2��+H2O��
�ܸ���ƷX�Ļ�ѧʽ�ǣ�CuSO4•5H2O����ѭ�����õ������ǣ�CO2��H2O��
�ʴ�Ϊ��CuSO4•5H2O��CO2��H2O��
�ݷ�Ӧ���з�����Ӧ��2NaHSO3?Na2S2O5+H2O�õ�Na2S2O5��Na��Sԭ����Ŀ֮��Ϊ1��1����2n��Na2CO3����n��SO2��=1��1����n��SO2����n��Na2CO3��=2��1��
�ʴ�Ϊ��2��1��
���� �����Խ����������Ʊ�Ϊ���壬���黯ѧ�������̡��Ȼ�ѧ����ʽ��д����Һ���ơ�������Ʊ���β�����������û�ѧ����ȣ�������ѧ���ķ���������ʵ�������Ŀ��飬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+Na+Br-S2- | B�� | Al3+Ca2+NO3-Cl- | ||
| C�� | Na+K+AlO2- NO3- | D�� | K+Na+SO42-S2O32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͨ����ˮ��ȥ�����е���Ȳ | |
| B�� | ���Ҵ����ȵ�170���Ʊ���ϩ | |
| C�� | ����Ũ��ˮ����˳�ȥ���еı��� | |
| D�� | �����������ƺ������ȥ���������е����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���ǣ�Z��W��M��Y��X | |
| B�� | �⻯��Ļ�ԭ�ԣ�Y��M��W | |
| C�� | Y��M�������ﶼ������������ | |
| D�� | X2Z�ĵ���ʽΪ��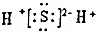 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ũ�� | c��NH3����mol/L�� | c��O2����mol/L�� | c��NO����mol/L�� |
| ��ʼ | 0.8 | 1.6 | 0 |
| ��2min | 0.6 | a | 0.2 |
| ��4min | 0.3 | 0.975 | 0.5 |
| ��6min | 0.3 | 0.975 | 0.5 |
| ��8min | 0.7 | 1.475 | 0.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe | B�� | Cu | C�� | ��ˮ | D�� | KSCN��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
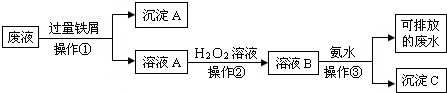
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
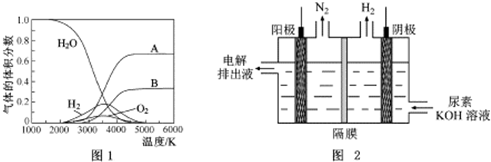
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com