
��ҵ���Ա�ϩΪԭ�Ͽ��Ƶ�һ����Ҫ�ϳ���IR��һ�ֺϳ���֬X��
��. ͬһ̼ԭ����������̼̼˫��ʱ���Ӳ��ȶ�
��1����һ�������£���ϩ�����������ʷ�Ӧ���� ��
a��H2O b. NaOH��Һ c. Br2��CCl4��Һ d. ����KMnO4��Һ
��2��A�����к˴Ź����������շ���________����
��3��A��C2H2�ϳ�D�Ļ�ѧ����ʽ��_______����Ӧ������________��
��4��E��F�о�����̼̼˫������ E�Ľṹ��ʽ��________��
��5��H�Ľṹ��ʽ��________��
��6��B�ķ���ʽ��C6H6O������NaOH��Һ��Ӧ��B��G��һ�������·�Ӧ���ɺϳ���֬X�Ļ�ѧ����ʽ��________��
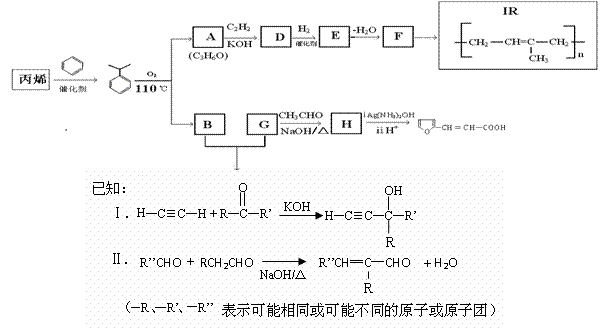
 ��7�� �ж���ͬ���칹�壬��������Ҫ�����________�֡�
��7�� �ж���ͬ���칹�壬��������Ҫ�����________�֡�
�� ������������������ȡ����
�� ȡ���������ǡ�ȩ�����Ȼ����������ǻ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯī���缫���������Һ��ϡH2SO4��K2SO4��Һ����CuCl2��Һ����CuSO4��Һ����KOH��Һ
(1)������������������������������(��ͬ������)Ϊ2��1����(�����)____________
______���������ĵ缫��Ӧʽ����____________________________________________��
�����ĵ缫��Ӧʽ����_______________________________________________��
�ܷ�Ӧ�Ļ�ѧ����ʽ����_____________________________________________��
(2)�����������������������������ҺpH��С����____��pH������______��(�����)
(3)һ���缫����������һ���缫�ݳ����壬����ҺpH���Լ�С����____________(�����)�����ܷ�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ����ȩ����ȩ��ɵĻ�����У���Ԫ�ص�����������9%������Ԫ�ص�����������(����)
A��16% B��37%
C��48% D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϵ�ⷨ�����������Է�ˮ���õ�����Ni��ԭ����ͼ��ʾ������˵������ȷ���ǣ� ��
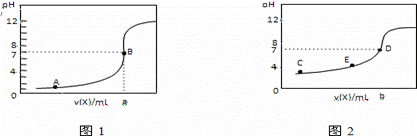 ��֪�� ��Ni2+����������Һ�з���ˮ��
��֪�� ��Ni2+����������Һ�з���ˮ��
�������ԣ�Ni2+����Ũ�ȣ���H+��Ni2+����Ũ�ȣ�
A��̼���Ϸ����ĵ缫��Ӧ��4OH����4e��==O2��+2H2O
B���������У�B��NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ���
C��Ϊ�����Ni�IJ��ʣ�����������Ҫ���Ʒ�ˮpH
D������ͼ��������Ĥȥ������A��B���Һϲ������ⷴӦ�ܷ���ʽ�����ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ������Ʒ֮һ����ͳ�Ĺ�ҵ�ϳɰ������ķ�Ӧԭ���ǣ�
N2��g����3H2��g�� 2NH3��g�� ��H����92.4 kJ/mol����500 �桢20 MPaʱ����N2��H2����һ��
2NH3��g�� ��H����92.4 kJ/mol����500 �桢20 MPaʱ����N2��H2����һ��
��1�����㷴Ӧ�ڵ�һ��ƽ��ʱ��ƽ�ⳣ��K�� ����������λС����
��2���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ�����____________����ȡ�Ĵ�ʩ��
________________________
��3������NH3��5��10 min��25��30min��45��50 minʱƽ����Ӧ���ʣ�ƽ����Ӧ���ʷֱ���v1��v2��v3��ʾ���Ӵ�С���д���Ϊ ��
��4����֪�������������������ˮ��Ϊ���ȷ�Ӧ���������ת�������¶�T1����ʱ�� ��t���ı仯����ͼ���������������䣬���ı��¶�ΪT2��T2< T1������ͼ�л����¶�T2�¼������ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ
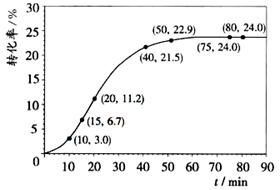
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҪʹAlCl3��Һ�е�Al3��ȫ�����������������õ��Լ���(����)
A��NaOH��Һ�������� ���� B��Ba(OH)2��Һ
C������ D����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ӷ���ʽ������д�ɻ�ѧ����ʽ��
(1)________��____Cu2������________��____Cu
_____________________________________________________��
(2)____Fe2O3��________����____Fe3����________
_____________________________________________________��
(3)____CaCO3��________����____CO2����________��_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����Ϊ��ȥ���к��е��������ӣ�ͨ�����õķ�����
A�����������÷�����
B�����Ҵ����÷�Һ©������
C��������������Һ���÷�Һ©������
D����Ũ��ˮ ���ù��˷�����
���ù��˷�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʵ����ʺ����ʵ�Ӧ�þ���ȷ����
A��SO2���������ԣ�������Ư��ֽ��
B��̿���л�ԭ�ԣ�һ���������ܽ��������軹ԭΪ��
C��BaCO3��BaSO4��������ˮ��������������
D��Al2O3���кܸߵ��۵㣬���������������ռ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com