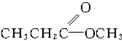��84����Һ����������Һ��Ӧ������ȡ��������Ӧ����ʽΪ��NaClO+NaCl+H
2SO
4 Na
2SO
4+Cl
2��+H
2O�� Ϊ̽�����������ʣ�ijͬѧ�����������ʾ��ʵ��װ��

��ش�
��1���Ӣ١��ڡ���װ����ѡ����ʵ�����װ�ã�A����
��
��
����д��ţ���
��2��װ��B��C�����ηŵ��Ǹ���ĺ�ɫ������ʪ��ĺ�ɫ������ʵ������и�ͬѧ����װ��B�еIJ���Ҳ��ɫ����ԭ�������
Cl2�л���������ˮ����
Cl2�л���������ˮ����
��˵����װ�ô������Ե�ȱ�ݣ�����������ĸĽ��ķ���
��A��B֮������ʢ��ŨH2SO4��ϴ��ƿ����ȥCl2�е�ˮ������
��A��B֮������ʢ��ŨH2SO4��ϴ��ƿ����ȥCl2�е�ˮ������
��
��3��Ϊ����֤�����������ԣ�������ͨNa
2SO
3��Һ�У�д��������Na
2SO
3��Һ��Ӧ�����ӷ���ʽ
SO32-+Cl2+H2O=SO42-+2Cl-+2H+
SO32-+Cl2+H2O=SO42-+2Cl-+2H+
��
��4��д������������������Һ��Ӧ�Ļ�ѧ����ʽ
Cl2+2NaOH=NaCl+NaClO+H2O
Cl2+2NaOH=NaCl+NaClO+H2O
��Ϊ��֤β�����պ����Һ�д��������ӣ���ȷ�IJ���Ϊ
ȡ������Һ���Թ��У��ȼ�HNO3����pHΪ���ԣ��ڼ�AgNO3��Һ�����а�ɫ�������ɣ�֤��ԭ��Һ����Cl-
ȡ������Һ���Թ��У��ȼ�HNO3����pHΪ���ԣ��ڼ�AgNO3��Һ�����а�ɫ�������ɣ�֤��ԭ��Һ����Cl-
��
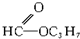
��5����֪RCH
2COOH��Cl
2�ں��״���ʱ����ȡ����Ӧ����RCHClCOOH�����з���ʽΪC
4H
8O
2������A���������±仯��

E�ķ���ʽΪC
8H
12O
4��E�ĺ˴Ź���������ʾֻ��һ���壮д��E�Ľṹ��ʽ
��




 ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�

 ��֪A�ķ���ʽΪC4H8O2������NaOH��Һ������Ӧ�IJ���������ת����ϵ������˵������ȷ���ǣ�������
��֪A�ķ���ʽΪC4H8O2������NaOH��Һ������Ӧ�IJ���������ת����ϵ������˵������ȷ���ǣ������� ������A��һ���������ķ���ʽΪC4H8O2������ͼת����ϵ���Իش��������⣮
������A��һ���������ķ���ʽΪC4H8O2������ͼת����ϵ���Իش��������⣮