
| A£® | NaBH4¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į | |
| B£® | NaBH4ŹĒŃõ»Æ¼Į£¬H2OŹĒ»¹Ō¼Į | |
| C£® | µČĪļÖŹµÄĮæµÄNaBH4”¢Na·Ö±šÓė×ćĮæĖ®·“Ó¦£¬NaBH4Éś³ÉµÄŃõ»Æ²śĪļ±ČNaÉŁ | |
| D£® | ±»Ńõ»ÆµÄŌŖĖŲÓė±»»¹ŌµÄŌŖĖŲÖŹĮæ±ČĪŖ1£ŗ1 |
·ÖĪö ·“Ó¦NaBH4+2H2O=NaBO2+4H2”üÖŠ£¬NaBH4ÖŠHŌŖĖŲ»ÆŗĻ¼ŪĪŖ-1¼Ū£¬Ė®ÖŠHŌŖĖŲ»ÆŗĻ¼ŪĪŖ+1¼Ū£¬¶žÕß·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉH2£¬2Na+2H2OØT2NaOH+H2”üÄĘĪŖ»¹Ō¼Į£¬Ė®ĪŖŃõ»Æ¼Į£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗA£®NaBH4ÖŠĒāŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬ĖłŅŌNaBH4ŹĒ»¹Ō¼Į£¬¹ŹA“ķĪó£»
B£®NaBH4ÖŠĒāŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬ĖłŅŌNaBH4ŹĒ»¹Ō¼Į£¬Ė®ÖŠĒāŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬ĖłŅŌĖ®ŹĒŃõ»Æ¼Į£¬¹ŹB“ķĪó£»
C£®µČĪļÖŹµÄĮæµÄNaBH4”¢Na·Ö±šÓė×ćĮæĖ®·“Ó¦£¬Ē°ÕßµÄŃõ»Æ²śĪļĪŖĒāĘų£¬ŗóÕßµÄŃõ»Æ²śĪļĪŖNaOH£¬ŌņNaBH4Éś³ÉµÄŃõ»Æ²śĪļ±ČNa¶ą£¬¹ŹC“ķĪó£»
D£®ŗĻ¼ŪÉżøßµÄŌŖĖŲŹĒNaBH4ÖŠµÄĒāŌŖĖŲ£¬±»Ńõ»Æ£¬Ė®ÖŠµÄĒāŌŖĖŲ±»»¹Ō£¬Ńõ»Æ¼ĮŗĶ»¹Ō¼ĮÖŠHŌŖĖŲµÄ»ÆŗĻ¼Ū±ä»ÆŹżÖµĻąĶ¬£¬ĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1£¬±»Ńõ»ÆµÄŌŖĖŲÓė±»»¹ŌµÄŌŖĖŲÖŹĮæ±ČĪŖ1£ŗ1£¬¹ŹDÕżČ·£®
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éŃõ»Æ»¹Ō·“Ó¦ÖŠµÄÓŠ¹ŲøÅÄīŗĶµē×Ó×ŖŅĘÖŖŹ¶£¬ĪŖøßĘµæ¼µć£¬²ąÖŲÓŚ»ł±¾øÅÄīµÄĄķ½āŗĶŌĖÓƵÄ漲飬עŅā“Ó»ÆŗĻ¼ŪµÄ½Ē¶Č½ā“šøĆĢā£¬½Ļ¼ņµ„£®


 Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø
Č«ÓÅ漵䵄ŌŖ¼ģ²ā¾ķ¼°¹éĄą×Üø“Ļ°ĻµĮŠ“š°ø ʷѧĖ«ÓžķĻµĮŠ“š°ø
ʷѧĖ«ÓžķĻµĮŠ“š°ø Š”ѧʌĩ³å“Ģ100·ÖĻµĮŠ“š°ø
Š”ѧʌĩ³å“Ģ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
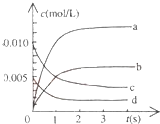 ŌŚ2LĆܱÕČŻĘ÷ÄŚ£¬800”ꏱ·“Ó¦£ŗ2NO£Øg£©+O2£Øg£©?2NO2£Øg£©ĢåĻµÖŠ£¬n£ØNO£©Ėꏱ¼äµÄ±ä»ÆČē±ķ£ŗ
ŌŚ2LĆܱÕČŻĘ÷ÄŚ£¬800”ꏱ·“Ó¦£ŗ2NO£Øg£©+O2£Øg£©?2NO2£Øg£©ĢåĻµÖŠ£¬n£ØNO£©Ėꏱ¼äµÄ±ä»ÆČē±ķ£ŗ| Ź±¼ä£Øs£© | 0 | 1 | 2 | 3 | 4 | 5 |
| n£ØNO£©£Ømol£© | 0.020 | 0.011 | 0.008 | 0.007 | 0.007 | 0.007 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
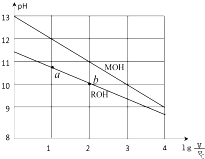 ŹŅĪĀĻĀ£¬½«ÅØ¶Č¾łĪŖ0.10mol/LĢå»ż¾łĪŖV0µÄMOHŗĶROHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶÖĮĢå»żV£¬pHĖę$lg\frac{V}{V_0}$µÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ĻĀĮŠŠšŹö“ķĪóµÄŹĒ£Ø””””£©
ŹŅĪĀĻĀ£¬½«ÅØ¶Č¾łĪŖ0.10mol/LĢå»ż¾łĪŖV0µÄMOHŗĶROHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶÖĮĢå»żV£¬pHĖę$lg\frac{V}{V_0}$µÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ĻĀĮŠŠšŹö“ķĪóµÄŹĒ£Ø””””£©| A£® | ¢Ū¢Ż | B£® | ¢Ł¢Ż | C£® | ¢Ł¢Ū | D£® | ¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŻĶČ”²Ł×÷Ź±£¬æÉŅŌŃ”ÓĆCCl4»ņ¾Ę¾«×÷ĪŖŻĶČ”¼Į“ÓµāĖ®ÖŠŻĶČ”µā | |
| B£® | Õō·¢²Ł×÷Ź±£¬Ó¦Ź¹»ģŗĻĪļÖŠµÄĖ®·ÖĶźČ«ÕōøÉŗ󣬲ÅÄÜĶ£Ö¹¼ÓČČ | |
| C£® | ·ÖŅŗ²Ł×÷Ź±£¬ĻĀ²ćŅŗĢå“Ó·ÖŅŗĀ©¶·ĻĀæŚ·Å³ö£¬ÉĻ²ćŅŗĢå“ÓĻĀæŚ·Å³öµ½ĮķŅ»øöÉÕ±ÖŠ | |
| D£® | ÕōĮó²Ł×÷Ź±£¬ŹÕ¼ÆĶźĮó·Öŗó£¬ĻČĶ£Ö¹¼ÓČČ£¬“ż»Öø“ŹŅĪĀŗóŌŁĶ£Ö¹ĶØĄäÄżĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{a}{288V}$mol/L | B£® | $\frac{125a}{36V}$mol/L | C£® | $\frac{125a}{18V}$mol/L | D£® | $\frac{125a}{54V}$mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·ÖŅŗ”¢ŻĶČ””¢ÕōĮó | B£® | ·ÖŅŗ”¢ÕōĮó”¢ŻĶČ” | C£® | ŻĶČ””¢ÕōĮ󔢷ÖŅŗ | D£® | ÕōĮó”¢ŻĶČ””¢·ÖŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £»
£»²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com