
��16�֣���֪����ͬһ��Ԫ�ص���������A��B��C��D 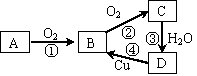 ��������ͼ��ʾ��ת����ϵ������AΪ���ʣ��ǹ�ҵ�Ϻϳɰ�����Ҫԭ�ϣ���
��������ͼ��ʾ��ת����ϵ������AΪ���ʣ��ǹ�ҵ�Ϻϳɰ�����Ҫԭ�ϣ���
��1������A�ĵ���ʽ�� ����ɵ���A��Ԫ��ΪX��XԪ�������ڱ���λ�ڵ� ���� �壬X���⻯��ĽṹʽΪ ����д���ϳɸ��⻯��ķ�Ӧ�Ļ�ѧ����ʽΪ ��
��2��D���ʵ������� ��D���ʵ�Ũ��Һ�������Դ���ɫ�����û�ѧ����ʽ��ʾ��ԭ��
��3����Ӧ���еĻ�ԭ���� ����д����Ӧ�ܵ����ӷ���ʽ ��
��4��������������B��C�Ĵ����ŷŻ�������Ⱦ��������дһ��B��C����Ļ������� ��
��5������ͼ����һʢ�д������������C���Թܵ�����ˮ�У�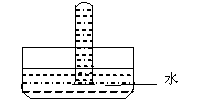 �����㹻����ʱ����Թ���ˮλ�����ĸ߶�Ϊ�Թܸ߶ȵ� ������ʢ��C ��O2���������Թܵ�����ˮ�У������㹻����ʱ����Թ������������СΪԭ���������֮һ����ԭ���������NO2��O2������ȿ����� ��
�����㹻����ʱ����Թ���ˮλ�����ĸ߶�Ϊ�Թܸ߶ȵ� ������ʢ��C ��O2���������Թܵ�����ˮ�У������㹻����ʱ����Թ������������СΪԭ���������֮һ����ԭ���������NO2��O2������ȿ����� ��
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��17�֣���֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������AΪ����ɫ���壬 F��ʹ��ɫʪ��ʯ����ֽ����ɫ������֮���ܷ������·�Ӧ��
�� A+H2O �� B+C �� C+F �� D �� D+NaOH E+F��+H2O
��1��д�����ǵĻ�ѧʽ��D ��F ��
��2��д���ٷ�Ӧ�Ļ�ѧ����ʽ�� ��
�˷�Ӧ���������� ����ԭ���� ��
��3��д����Ӧ�۵����ӷ���ʽ�� ��
��4����ҵ����C�Ĺ�����������һ����Ӧ����F������������B��H2O��д���ò���Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ��ɽ��ѧ��һ��ѧ��3���¿���ѧ�Ծ� ���ͣ������
��15�֣���֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ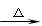
�� A + H2O �� B + C �� C + F �� D �� D + NaOH F + E + H2O
��1��д�����ǵĻ�ѧʽ��A ��B �� D ��
E ��F ��
��2��д��������Ӧ�����ӷ���ʽ����ָ��Ӧ�ٵ��������ͻ�ԭ�����ʵ����ȡ�
�� ���������뻹ԭ�����ʵ�����Ϊ�� ��
�� ��
�� ��
��3����ҵ����C�Ĺ�����������һ����Ӧ����F������������B��H2O��д���ò���Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com