
| |||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����д���¿հף�
(1)B�ĵ���ʽΪ____________��D���ӵĿռ乹��Ϊ____________��
(2)д����Ӧ�٢ڵĻ�ѧ����ʽ��
��___________________________________________��
��___________________________________________��
(3)��Ӧ�۵����ӷ���ʽΪ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A���ȷֽ�õ���ͬ���ʵ�����B��C��D(ͼ���в���������δд��)��
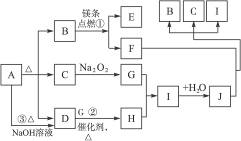
����д���¿հף�
(1)B�ĵ���ʽΪ____________��D���ӵĿռ乹��Ϊ____________��
(2)д����Ӧ�٢ڵĻ�ѧ����ʽ��
��___________________________________________��
��___________________________________________��
(3)��Ӧ�۵����ӷ���ʽΪ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��A~J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����
����д���¿հף�
��1��A�Ļ�ѧʽ ��B�ĵ���ʽ ��
��2��H��I�Ի�����ɵ�Σ���� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
D+ G��H ��
F+J��B +C +I ��
��4��д��A+NaOH��D�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣���ͼ��A~J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A�ֽ�õ������ʵ�����B��C��D����֪B��DΪ��������̬�����CΪ������Һ̬�����ͼ���в���������δ�����
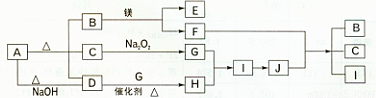
����д���¿հף�
��1��A�Ļ�ѧʽ ��B�ĵ���ʽ ��
��2��H��I�Ի�����ɵ�Σ���� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
D+ G��H ��
F+J��B +C +I ��
��4��д��A+NaOH��D�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A~J�ֱ������ط�Ӧ�е�һ�����ʣ���֪A���ȷֽ�õ���ͬ���ʵ�����B��C��D��ͼ���в���������δд������
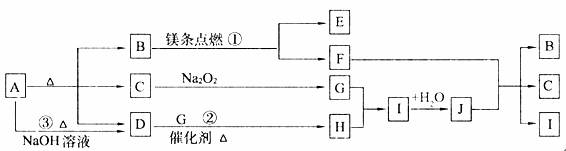
����д���¿հף�
��1��B�ĵ���ʽΪ_________________��D���ӵĿռ乹��Ϊ_______________��
��2��д����Ӧ�١��ڵĻ�ѧ����ʽ��
��___________________________________________________________��
��__________________________________________________________��
��3����Ӧ�۵����ӷ���ʽΪ��____________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com