
|
ĻĀĮŠČČ»Æѧ·½³ĢŹ½»ņĖµ·ØÕżČ·µÄŹĒ | |
| [””””] | |
A£® |
¼×ĶéµÄČ¼ÉÕČČĪŖ¦¤H£½£890 kJ”¤mol£1£¬Ōņ¼×ĶéČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½æɱķŹ¾ĪŖ£ŗCH4(g)£«2O2(g) |
B£® |
500”ę”¢30 MPaĻĀ£¬½«0.5 mol””N2ŗĶ1.5 mol””H2ÖĆÓŚĆܱյÄČŻĘ÷ÖŠ³ä·Ö·“Ӧɜ³ÉNH3(g)£¬·ÅČČ19.3 kJ£¬ĘäČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ N2(g)£«3H2(g) |
C£® |
ŅŃÖŖ£ŗH2(g)£«F2(g)£½2HF(g)£»¦¤H£½£270 kJ/mol£¬Ōņ1 molĒāĘųÓė1 mol·śĘų·“Ӧɜ³É2 molŅŗĢ¬·ś»ÆĒā·Å³öµÄČČĮæŠ”ÓŚ270 kJ |
D£® |
ŌŚCÖŠĻąĶ¬Ģõ¼žĻĀ£¬2 mol””HFĘųĢåµÄÄÜĮæŠ”ÓŚ1 molĒāĘųÓė1 mol·śĘųµÄÄÜĮæ×ÜŗĶ |

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ĘšŹ¼ÅØ¶Č | ¼× | ŅŅ | ±ū |
| c£ØH2£©/mol/L | 0.010 | 0.020 | 0.020 |
| c£ØCO2£©/mol/L | 0.010 | 0.010 | 0.020 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø7·Ö£©»Æѧ¼üµÄ¼üÄÜŹĒÖøĘųĢ¬Ō×Ó¼äŠĪ³É1mol»Æѧ¼üŹ±ŹĶ·ÅµÄÄÜĮ攣Čē£ŗH(g)£«I(g)”śH£I(g)£«297KJ ¼“H£I¼üµÄ¼üÄÜĪŖ297kJ/mol£¬Ņ²æÉŅŌĄķ½āĪŖĘĘ»µ1mol H£I¼üŠčŅŖĪüŹÕ297KJµÄČČĮ攣»Æѧ·“Ó¦µÄ·¢ÉśæÉŅŌ擳ɾɻÆѧ¼üµÄĘĘ»µŗĶŠĀ»Æѧ¼üµÄŠĪ³É”£ĻĀ±ķŹĒŅ»Š©¼üÄÜŹż¾Ż”££Øµ„Ī»£ŗkJ/mol£©
|
| ¼üÄÜ |
| ¼üÄÜ |
| ¼üÄÜ |
| H£H | 436 | Cl£Cl | 243 | H£Cl | 432 |
| S£½S | 255 | H£S | 339 | C£F | 427 |
| C£Cl | 330 | C£I | 218 | H£F | 565 |
| C£O | 347 | H£O | 464 | Si”ŖSi | 176 |
| Si”ŖO | 460 | O=O | 497 |
|
|
ŌĶĮÉĻŹöŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øł¾Ż±ķÖŠŹż¾ŻÅŠ¶ĻCCl4µÄĪČ¶ØŠŌ £ØĢī”°“óÓŚ”±»ņ”°Š”ÓŚ”±£©CF4µÄĪČ¶ØŠŌ”£ŹŌŌ¤²āC£Br¼üµÄ¼üÄÜ·¶Ī§_________< C£Br¼üÄÜ <__________
£Ø2£©ÓŠČĖČĻĪŖ£ŗH£O¼üµÄ¼üÄÜ“óÓŚH£S¼üµÄ¼üÄÜ£¬ĖłŅŌH2OµÄČŪ·ŠµćøßÓŚH2SµÄČŪ·Šµć”£ÄćŹĒ·ńŌŽĶ¬ÕāÖÖ¹Ūµć£æČē²»ŌŽĶ¬£¬ĒėĖµ³öÄćµÄ½āŹĶ”£
£Ø3£©ŅŃÖŖH2O(l)£½H2O(g) ¦¤H£½+44kJ/mol,ĒėŠ“³ö±ķŹ¾ĒāĘųČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø16·Ö£©ŅŌĆŗĪŖŌĮĻ£¬¾¹ż»Æѧ¼Ó¹¤Ź¹Ćŗ×Ŗ»ÆĪŖĘųĢ唢ŅŗĢ唢¹ĢĢåČ¼ĮĻŅŌ¼°ø÷Öֻƹ¤²śĘ·µÄ¹¤Ņµ½ŠĆŗ»Æ¹¤”£
£Ø1£©½«Ė®ÕōĘųĶعżŗģČȵÄĢ¼¼“æɲśÉśĖ®ĆŗĘų”£·“Ó¦ĪŖ£ŗ
C(s)£«H2O(g)CO(g)£«H2(g) ¦¤H=£«131.3kJ•mol-1£¬
¢ŁøĆ·“Ó¦ŌŚ³£ĪĀĻĀ ×Ō·¢½ųŠŠ£ØĢī”°ÄÜ”±Óė”°²»ÄÜ”±£©£»
¢ŚŗćĪĀ£¬ŌŚČŻ»żæɱäµÄĆܱÕČŻĘ÷ÖŠ£¬½ųŠŠČēÉĻæÉÄę·“Ó¦”£Ņ»¶ĪŹ±¼äŗó£¬ĻĀĮŠĪļĄķĮæ²»·¢Éś±ä»ÆŹ±£¬ÄܱķĆ÷øĆ·“Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄÓŠ
¢ń»ģŗĻĘųĢåµÄĆÜ¶Č£» ¢ņČŻĘ÷ÄŚĘųĢåµÄŃ¹Ē棻
¢ó»ģŗĻĘųĢåµÄ×ÜĪļÖŹµÄĮ棻 ¢ōCOĪļÖŹµÄĮæÅضČ
A£®Ö»ÓŠ¢ō B£®Ö»ÓŠ¢ńŗĶ¢ō C£®Ö»ÓŠ¢ņŗĶ¢ó D£®¢ń”¢¢óŗĶ¢ō
£Ø2£©Ė®ĆŗĘųŌŁ½ųŅ»²½·“Ó¦æÉÖĘČ”ĒāĘų”£·“Ó¦ĪŖH2O(g)+CO(g) H2(g)+CO2(g)£¬Ä³ĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= 4/9”£øĆĪĀ¶ČĻĀŌŚ¼×”¢ŅŅ”¢±ūČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ£¬Ö»Ķ¶ČėH2(g)ŗĶCO2(g)£¬ĘäĘšŹ¼ÅضČČēĻĀ±ķĖłŹ¾”£ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ ”£
| ĘšŹ¼ÅØ¶Č | ¼× | ŅŅ | ±ū |
| c(H2)/mol/L | 0.010 | 0.020 | 0.020 |
| c(CO2)/mol/L | 0.010 | 0.010 | 0.020 |
A£®·“Ó¦æŖŹ¼Ź±£¬±ūÖŠµÄ·“Ó¦ĖŁĀŹ×īæģ£¬¼×ÖŠµÄ·“Ó¦ĖŁĀŹ×īĀż
B£®Ę½ŗāŹ±£¬¼×ÖŠŗĶ±ūÖŠ£Č2µÄ×Ŗ»ÆĀŹ¾łŹĒ60£„
C£®Ę½ŗāŹ±£¬±ūÖŠ£ć(CO2)ŹĒ¼×ÖŠµÄ2±¶£¬ŹĒ0.012mol/L
D£®Ę½ŗāŹ±£¬ŅŅÖŠCO2µÄ×Ŗ»ÆĀŹ“óÓŚ60%
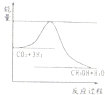
£Ø3£©ÄæĒ°¹¤ŅµÉĻÓŠŅ»ÖÖ·½·ØŹĒÓĆCO2Ą“Éś²ś¼×“¼”£Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g)CH3OH(g)£«H2O(g) £¬ÓŅĶ¼±ķŹ¾øĆ·“Ó¦½ųŠŠ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ•mol”Ŗ1)µÄ±ä»Æ”£ŌŚĢå»żĪŖ1 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2·“Ó¦
¢ŁĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹c (CH3OH)Ōö“óµÄŹĒ ”£
A£®ÉżøßĪĀ¶Č
B£®³äČėHe(g)£¬Ź¹ĢåĻµŃ¹ĒæŌö“ó
C£®½«H2O(g)“ÓĢåĻµÖŠ·ÖĄė³öĄ“
D£®ŌŁ³äČė1molCO2ŗĶ3molH2
¢ŚŌŚĪĀ¶ČT1Ź±£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆn(H2) =2.4 mol£»ĘäĖüĢõ¼ž²»±ä£¬ŌŚĪĀ¶ČT2Ź±£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆn(CO2) =0.82 mol£¬ŌņT2 T1”££ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©£¬
£Ø4£©ŌŚŅ»¶ØĢõ¼žĻĀæĘѧ¼Ņ“ÓŃĢµĄĘųÖŠ·ÖĄė³öCO2ÓėĢ«ŃōÄܵē³Ųµē½āĖ®²śÉśµÄH2ŗĻ³É¼×“¼”£CH3OH”¢H2µÄČ¼ÉÕČČ·Ö±šĪŖ£ŗ”÷H£½£725.5kJ/mol”¢”÷H£½£285.8kJ/mol”£
¢ŁŠ“³ö¹¤ŅµÉĻŅŌCO2”¢H2ŗĻ³ÉCH3OHŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½£ŗ £»
¢ŚøĆ×Ŗ»ÆµÄ»ż¼«ŅāŅåŹĒ £»
¢ŪÓŠČĖĢį³ö£¬æÉŅŌÉč¼Ę·“Ó¦CO2£½C£«O2£Ø”÷H£¾0”¢”÷S£¼0£©Ą“Ļū³żCO2¶Ō»·¾³µÄÓ°Ļģ”£ĒėÄćÅŠ¶ĻŹĒ·ńæÉŠŠ²¢Ėµ³öĄķÓÉ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģĖÄ“ØŹ”ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø7·Ö£©»Æѧ¼üµÄ¼üÄÜŹĒÖøĘųĢ¬Ō×Ó¼äŠĪ³É1mol»Æѧ¼üŹ±ŹĶ·ÅµÄÄÜĮ攣Čē£ŗH(g)£«I(g)”śH£I(g)£«297KJ ¼“H£I¼üµÄ¼üÄÜĪŖ297kJ/mol£¬Ņ²æÉŅŌĄķ½āĪŖĘĘ»µ1mol H£I¼üŠčŅŖĪüŹÕ297KJµÄČČĮ攣»Æѧ·“Ó¦µÄ·¢ÉśæÉŅŌ擳ɾɻÆѧ¼üµÄĘĘ»µŗĶŠĀ»Æѧ¼üµÄŠĪ³É”£ĻĀ±ķŹĒŅ»Š©¼üÄÜŹż¾Ż”££Øµ„Ī»£ŗkJ/mol£©
|
|
¼üÄÜ |
|
¼üÄÜ |
|
¼üÄÜ |
|
H£H |
436 |
Cl£Cl |
243 |
H£Cl |
432 |
|
S£½S |
255 |
H£S |
339 |
C£F |
427 |
|
C£Cl |
330 |
C£I |
218 |
H£F |
565 |
|
C£O |
347 |
H£O |
464 |
Si”ŖSi |
176 |
|
Si”ŖO |
460 |
O=O |
497 |
|
|
ŌĶĮÉĻŹöŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øł¾Ż±ķÖŠŹż¾ŻÅŠ¶ĻCCl4µÄĪČ¶ØŠŌ £ØĢī”°“óÓŚ”±»ņ”°Š”ÓŚ”±£©CF4µÄĪČ¶ØŠŌ”£ŹŌŌ¤²āC£Br¼üµÄ¼üÄÜ·¶Ī§_________< C£Br¼üÄÜ <__________
£Ø2£©ÓŠČĖČĻĪŖ£ŗH£O¼üµÄ¼üÄÜ“óÓŚH£S¼üµÄ¼üÄÜ£¬ĖłŅŌH2OµÄČŪ·ŠµćøßÓŚH2SµÄČŪ·Šµć”£ÄćŹĒ·ńŌŽĶ¬ÕāÖÖ¹Ūµć£æČē²»ŌŽĶ¬£¬ĒėĖµ³öÄćµÄ½āŹĶ”£
£Ø3£©ŅŃÖŖH2O(l)£½H2O(g) ¦¤H£½+44kJ/mol,ĒėŠ“³ö±ķŹ¾ĒāĘųČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ½ĖÕŹ”ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©ŅŌĆŗĪŖŌĮĻ£¬¾¹ż»Æѧ¼Ó¹¤Ź¹Ćŗ×Ŗ»ÆĪŖĘųĢ唢ŅŗĢ唢¹ĢĢåČ¼ĮĻŅŌ¼°ø÷Öֻƹ¤²śĘ·µÄ¹¤Ņµ½ŠĆŗ»Æ¹¤”£
£Ø1£©½«Ė®ÕōĘųĶعżŗģČȵÄĢ¼¼“æɲśÉśĖ®ĆŗĘų”£·“Ó¦ĪŖ£ŗ
C(s)£«H2O(g)
 CO(g)£«H2(g) ¦¤H=£«131.3
kJ•mol-1£¬
CO(g)£«H2(g) ¦¤H=£«131.3
kJ•mol-1£¬
¢ŁøĆ·“Ó¦ŌŚ³£ĪĀĻĀ ×Ō·¢½ųŠŠ£ØĢī”°ÄÜ”±Óė”°²»ÄÜ”±£©£»
¢ŚŗćĪĀ£¬ŌŚČŻ»żæɱäµÄĆܱÕČŻĘ÷ÖŠ£¬½ųŠŠČēÉĻæÉÄę·“Ó¦”£Ņ»¶ĪŹ±¼äŗó£¬ĻĀĮŠĪļĄķĮæ²»·¢Éś±ä»ÆŹ±£¬ÄܱķĆ÷øĆ·“Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄÓŠ
¢ń»ģŗĻĘųĢåµÄĆÜ¶Č£» ¢ņČŻĘ÷ÄŚĘųĢåµÄŃ¹Ē棻
¢ó»ģŗĻĘųĢåµÄ×ÜĪļÖŹµÄĮ棻 ¢ōCOĪļÖŹµÄĮæÅضČ
A£®Ö»ÓŠ¢ō B£®Ö»ÓŠ¢ńŗĶ¢ō C£®Ö»ÓŠ¢ņŗĶ¢ó D£®¢ń”¢¢óŗĶ¢ō
£Ø2£©Ė®ĆŗĘųŌŁ½ųŅ»²½·“Ó¦æÉÖĘČ”ĒāĘų”£·“Ó¦ĪŖH2O(g)+CO(g) H2(g)+CO2(g)£¬Ä³ĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= 4/9”£øĆĪĀ¶ČĻĀŌŚ¼×”¢ŅŅ”¢±ūČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ£¬Ö»Ķ¶ČėH2(g)ŗĶCO2(g)£¬ĘäĘšŹ¼ÅضČČēĻĀ±ķĖłŹ¾”£ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ ”£
H2(g)+CO2(g)£¬Ä³ĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= 4/9”£øĆĪĀ¶ČĻĀŌŚ¼×”¢ŅŅ”¢±ūČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ£¬Ö»Ķ¶ČėH2(g)ŗĶCO2(g)£¬ĘäĘšŹ¼ÅضČČēĻĀ±ķĖłŹ¾”£ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ ”£
|
ĘšŹ¼ÅØ¶Č |
¼× |
ŅŅ |
±ū |
|
c(H2)/mol/L |
0.010 |
0.020 |
0.020 |
|
c(CO2)/mol/L |
0.010 |
0.010 |
0.020 |
A£®·“Ó¦æŖŹ¼Ź±£¬±ūÖŠµÄ·“Ó¦ĖŁĀŹ×īæģ£¬¼×ÖŠµÄ·“Ó¦ĖŁĀŹ×īĀż
B£®Ę½ŗāŹ±£¬¼×ÖŠŗĶ±ūÖŠ£Č2µÄ×Ŗ»ÆĀŹ¾łŹĒ60£„
C£®Ę½ŗāŹ±£¬±ūÖŠ£ć(CO2)ŹĒ¼×ÖŠµÄ2±¶£¬ŹĒ0.012mol/L
D£®Ę½ŗāŹ±£¬ŅŅÖŠCO2µÄ×Ŗ»ÆĀŹ“óÓŚ60%
£Ø3£©ÄæĒ°¹¤ŅµÉĻÓŠŅ»ÖÖ·½·ØŹĒÓĆCO2Ą“Éś²ś¼×“¼”£Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g) CH3OH(g)£«H2O(g) £¬ÓŅĶ¼±ķŹ¾øĆ·“Ó¦½ųŠŠ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ•mol”Ŗ1)µÄ±ä»Æ”£ŌŚĢå»żĪŖ1 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2·“Ó¦
CH3OH(g)£«H2O(g) £¬ÓŅĶ¼±ķŹ¾øĆ·“Ó¦½ųŠŠ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ•mol”Ŗ1)µÄ±ä»Æ”£ŌŚĢå»żĪŖ1 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČė1mol CO2ŗĶ3mol H2·“Ó¦

¢ŁĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹c (CH3OH)Ōö“óµÄŹĒ ”£
A£®ÉżøßĪĀ¶Č
B£®³äČėHe(g)£¬Ź¹ĢåĻµŃ¹ĒæŌö“ó
C£®½«H2O(g)“ÓĢåĻµÖŠ·ÖĄė³öĄ“
D£®ŌŁ³äČė1mol CO2ŗĶ3mol H2
¢ŚŌŚĪĀ¶ČT1Ź±£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆn(H2) = 2.4 mol£»ĘäĖüĢõ¼ž²»±ä£¬ŌŚĪĀ¶ČT2Ź±£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬²āµĆn(CO2) = 0.82 mol£¬ŌņT2 T1”££ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©£¬
£Ø4£©ŌŚŅ»¶ØĢõ¼žĻĀæĘѧ¼Ņ“ÓŃĢµĄĘųÖŠ·ÖĄė³öCO2ÓėĢ«ŃōÄܵē³Ųµē½āĖ®²śÉśµÄH2ŗĻ³É¼×“¼”£CH3OH”¢H2µÄČ¼ÉÕČČ·Ö±šĪŖ£ŗ”÷H£½£725.5kJ/mol”¢”÷H£½£285.8kJ/mol”£
¢ŁŠ“³ö¹¤ŅµÉĻŅŌCO2”¢H2ŗĻ³ÉCH3OHŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½£ŗ £»
¢ŚøĆ×Ŗ»ÆµÄ»ż¼«ŅāŅåŹĒ £»
¢ŪÓŠČĖĢį³ö£¬æÉŅŌÉč¼Ę·“Ó¦CO2£½C£«O2£Ø”÷H£¾0”¢”÷S£¼0£©Ą“Ļū³żCO2¶Ō»·¾³µÄÓ°Ļģ”£ĒėÄćÅŠ¶ĻŹĒ·ńæÉŠŠ²¢Ėµ³öĄķÓÉ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com