
ͨ���۲컯ѧʵ�����������ɳ���ȷ�Ľ��ۣ���ѧϰ��ѧ��ѧ������ļ���֮һ��������ʵ������ó���ȷ���۵���
| ѡ�� | ʵ������ | ���� |
| A | ����ͬ��С��һ������þ�ֱ�Ͷ����ͬŨ�ȵ�NaOH��Һ�У����ܽ������ݷų�����þû���κα仯 | ���Ľ����Ա�þǿ |
| B | ȡһ���������þƾ��Ƶ�ȼ������ֻ�ۻ���Һ���û������ | ������ȼ�� |
| C | ���Ȼ����Һ��Ͷ��һ��Ƭ����Ƭ�ϲ����������� | �������ǰ��� |
| D | ���Ȼ�����Һ����εμ�NaOH��Һ���������Ȳ�����ɫ��������������ܽ⣬��������ȫ��ʧ | ��������������ǿ����Һ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������и�װ��ͼ�������У�����ȷ����
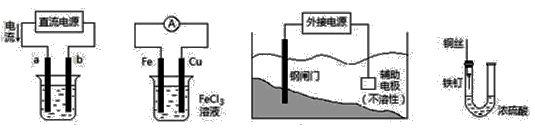
�� �� �� ��
A����װ�âپ���ͭ����a��Ϊ��ͭ���������ҺΪCuSO4��Һ
B��װ�âڵ��ܷ�Ӧ�ǣ�Cu��2Fe3���� Cu2����2Fe2��
C��װ�â��и�բ��Ӧ����ӵ�Դ�ĸ�������
D��װ�â��е���������û����ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ƻ��֭������ϲ�������ϣ����ڴ������к���Fe2+���ӣ���ե��ƻ��֭�ڿ����л��ɵ���ɫ��Ϊ�ػ�ɫ��ե֭ʱ����ά����C����Ч��ֹ������������˵��ά����C���У� ��
A�������� B����ԭ�� C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ƺ�̼���ƵĻ����4.96 g�м���������4.80%�����ᣨ�ܶ�Ϊ1.02 g/mL������ַ�Ӧ���ڱ�״�����ռ���0.448 L������̼������
��1���������̼���Ƶ����ʵ�����
��2��������������Ƶ�����������������λС������
��3����������ʵ���Ũ�ȣ�������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���������������ȷ����
A������ԭ��ǿ��������ǿ�����Կ�ͨ�����ȷ�Ӧұ�����۵����
B�����ڿ����м�����ʴ����������Ʒ��ʹ��ʱ��ܶ�
C��������ϡ���ḯʴ����������ϡ����������Ʋ۳�
D�����ĵ����Ա�ͭ������ǿ�����Գ�����������ߡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3��Һ�к��е�Mg2����K���������Ӳ������ܼ���AlCl3����ʧ��
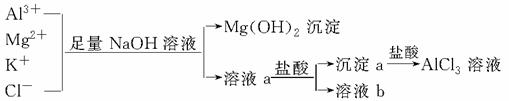
����˵����ȷ����
A��NaOH��Һ�����ð�ˮ������
B����Һa�к���Al3����K����Cl����Na����OH��
C����Һb��ֻ����NaCl
D������Һa�еμ������������Һ��pH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ë��պȡ����30% FeCl3��Һ��ͭƬ��дһ����Cu���֣�����Ƭ�̣�������ˮ��ͭƬ�ϵ���Һ�嵽С�ձ��У�����˵����ȷ����
A���ձ��е���Һ�ʻ�ɫ
B��ͭƬ���κα仯
C��ͭƬ���а��ݵġ�Cu����
D�������˷�Ӧ��Fe3����Cu===Cu2����Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǷŴ��ط�����Դ����ҵ����Ҳ���������á�ij��ѧ��ȤС����ʵ�������÷����ĺ���������ͭ�ĺϽ���ȡ��������Һ������ͭ���������(Fe2O3)��ʵ�鷽�����£�

(1)д����ҺA�м��������������������Ӧ�����ӷ���ʽ��________________________________________________________________________��
(2)��֪Fe(OH)3������pH��3��4����ҺCͨ������pH����ʹFe3��������ȫ�����������п�����������ҺC��pH���Լ���________(�����)��
A��ͭ�ۡ��������������� B����ˮ
C��������ͭ D����ʽ̼��ͭ
(3)���£�����ҺC�н������Ӿ�Ϊ1 mol��L��1��Ksp[Fe(OH)3]��4.0��10��38��Ksp[Cu(OH)2]��2.2��10��20������pH��4����Һ��c(Fe3��)��_____________________________________��
��ʱ________Cu(OH)2��������(��С����ޡ�)��
(4)��20 mL Al2(SO4)3��Һ������ʵ���Ũ�ȵ�Ba(OH)2��Һ70 mL��ϣ���Ӧ�����ӷ���ʽΪ__________________________________________________________________��
(5)��0.1 L�Ļ������Һ�У�c(HNO3)��2 mol��L��1��c(H2SO4)��3 mol��L��1����0.3 mol��ͭ������ȳ�ַ�Ӧ����ԭ��HNO3�����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£����淴Ӧ2A=B��3C������������״̬�д���ƽ��״̬����(����)
����Ӧ�ٶ�=�淴Ӧ�ٶ�
A��vA��2mol/(L��min)����vB��2mol/(L��min)
B��vA��2mol/(L��min)����vC��2mol/(L��min)
C��vA��1mol/(L��min)����vB��2mol/(L��min)
D��vA��1mol/(L��min)����vC��1.5mol/(L��min)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com