
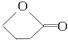
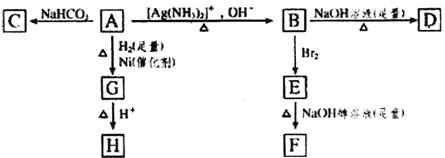
HŹĒ»·×“»ÆŗĻĪļC4H6O2£¬FµÄĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ”£
(1)»ÆŗĻĪļAŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ__________”£
(2)BŌŚĖįŠŌĢõ¼žĻĀÓėBr2·“Ó¦µĆµ½E£¬EŌŚ×ćĮæµÄĒāŃõ»ÆÄĘ“¼ČÜŅŗ×÷ÓĆĻĀ×Ŗ±äĪŖF£¬ÓÉE×Ŗ±äĪŖFŹ±·¢ÉśĮ½ÖÖ·“Ó¦£¬Ęä·“Ó¦ĄąŠĶ·Ö±šŹĒ__________”£
(3)DµÄ½į¹¹¼ņŹ½ŹĒ_____________________________________________”£
(4)1 mol AÓė2 mol H2·“Ӧɜ³É1 mol E£¬Ęä·“Ó¦·½³ĢŹ½ŹĒ__________________________”£
(5)ÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ŹĒ____________________________”£
½āĪö£ŗAÄÜÓėŅų°±ČÜŅŗ·“Ó¦£¬ĖµĆ÷AÖŠÓŠ”ŖCHO£¬ÓÖÄÜÓėNaHCO3·“Ó¦£¬ŌņAÖŠ»¹ÓŠ”ŖCOOH”£µ±AÓėH2·“Ӧɜ³ÉGŹ±£¬”ŖCHO×Ŗ±äĪŖ”ŖCH2OH£¬¼ŁÉč¼ČÓŠ”ŖCOOH£¬ÓÖÓŠ”ŖOH£¬ŌŚĖįŠŌĢõ¼žĻĀ£¬·¢Éśõ„»Æ·“Ó¦£¬Éś³É»·ÄŚõ„£ŗC4H6O2”£ÓÖŅņĪŖFÖŠĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ(ĖµĆ÷FÖŠ“ęŌŚC”ŌC)£¬Ōņ4øöCŌ×ÓĪŽÖ§Į“½į¹¹”£ĖłŅŌHĪŖ![]() £¬GĪŖCH2OHCH2CH2CH2COOH”£øł¾Ż(4)ÖŠ1 mol AÓė2 mol H2·“Ó¦£¬ĘäÖŠ”ŖCHOŠč1 mol H2£¬ŌņĖµĆ÷AÖŠ»¹ÓŠŅ»øöC=C£¬Ņņ“ĖAĪŖOHC”ŖCH=CHCOOH£¬BĪŖHOOC”ŖCH=CH”ŖCOOH£¬DĪŖNaOOC”ŖCH=CH”ŖCOONa£¬CĪŖOHC”ŖCH=CH”ŖCOONaŌŚ“¼¼īČÜŅŗÖŠ£¬·¢ÉśĻūČ„ÓėÖŠŗĶ·“Ó¦ŗó£¬Éś³ÉµÄFĪŖNaOO”ŖC”ŌC”ŖCOONa£¬ÓÖŅņĪŖ”ŖCHOÓė”ŖCOOH±ŲŠėŌŚĢ¼Į“Į“¶Ė£¬½«”ŖCHO(»ņ”ŖCOOH)æ“×÷ĪŖČ”“ś»łĶÅ£¬æÉŅʵ½C=CµÄĮķŅ»øöĢ¼Ō×ÓÉĻ£¬ĖłŅŌAµÄĶ¬·ÖŅģ¹¹Ģå(¾ßÓŠĻąĶ¬¹ŁÄÜĶÅ)ĪŖ
£¬GĪŖCH2OHCH2CH2CH2COOH”£øł¾Ż(4)ÖŠ1 mol AÓė2 mol H2·“Ó¦£¬ĘäÖŠ”ŖCHOŠč1 mol H2£¬ŌņĖµĆ÷AÖŠ»¹ÓŠŅ»øöC=C£¬Ņņ“ĖAĪŖOHC”ŖCH=CHCOOH£¬BĪŖHOOC”ŖCH=CH”ŖCOOH£¬DĪŖNaOOC”ŖCH=CH”ŖCOONa£¬CĪŖOHC”ŖCH=CH”ŖCOONaŌŚ“¼¼īČÜŅŗÖŠ£¬·¢ÉśĻūČ„ÓėÖŠŗĶ·“Ó¦ŗó£¬Éś³ÉµÄFĪŖNaOO”ŖC”ŌC”ŖCOONa£¬ÓÖŅņĪŖ”ŖCHOÓė”ŖCOOH±ŲŠėŌŚĢ¼Į“Į“¶Ė£¬½«”ŖCHO(»ņ”ŖCOOH)æ“×÷ĪŖČ”“ś»łĶÅ£¬æÉŅʵ½C=CµÄĮķŅ»øöĢ¼Ō×ÓÉĻ£¬ĖłŅŌAµÄĶ¬·ÖŅģ¹¹Ģå(¾ßÓŠĻąĶ¬¹ŁÄÜĶÅ)ĪŖ![]() ”£
ӣ
“š°ø£ŗ(1)Č©»ł”¢ōČ»ł”¢Ģ¼Ģ¼Ė«¼ü (2)ĻūČ„·“Ó¦”¢ÖŠŗĶ·“Ó¦
(3)NaOOC”ŖCH=CH”ŖCOONa



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻĶ¼Ź¾ĢīæÕ£ŗ
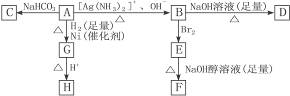
HŹĒ»·×“»ÆŗĻĪļC4H6O2£¬FµÄĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ”£
£Ø1£©»ÆŗĻĪļAŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ__________”£
£Ø2£©BŌŚĖįŠŌĢõ¼žĻĀÓėBr2·“Ó¦µĆµ½E£¬EŌŚ×ćĮæµÄĒāŃõ»ÆÄĘ“¼ČÜŅŗ×÷ÓĆĻĀ×Ŗ±äĪŖF£¬ÓÉE×Ŗ±äĪŖFŹ±·¢ÉśĮ½ÖÖ·“Ó¦£¬Ęä·“Ó¦ĄąŠĶ·Ö±šŹĒ__________”£
£Ø3£©DµÄ½į¹¹¼ņŹ½ŹĒ_____________________________________________”£
£Ø4£©1 mol AÓė2 mol H2·“Ӧɜ³É1 mol E£¬Ęä·“Ó¦·½³ĢŹ½ŹĒ__________________________”£
£Ø5£©ÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ŹĒ____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻĶ¼Ź¾ĢīæÕ
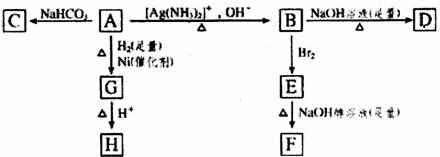
HŹĒ»·×“»ÆŗĻĪļC4H6O2 FµÄĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ
£Ø1£©»ÆŗĻĪļAŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ ”£
£Ø2£©BŌŚĖįŠŌĢõ¼žĻĀÓėBr2·“Ó¦µĆµ½E£¬EŌŚ×ćĮæµÄĒāŃõ»ÆÄĘ“¼ČÜŅŗ×÷ÓĆĻĀ×Ŗ±äĪŖF£¬ÓÉE×Ŗ±äĪŖFŹ±·¢ÉśĮ½ÖÖ·“Ó¦£¬Ęä·“Ó¦ĄąŠĶ·Ö±šŹĒ ”£
£Ø3£©DµÄ½į¹¹¼ņŹ½ŹĒ ”£
£Ø4£©1mol AÓė2mol H2·“Ӧɜ³É1mol G£¬Ęä·“Ó¦·½³ĢŹ½ŹĒ ”£
£Ø5£©ÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻĶ¼Ź¾ĢīæÕ
HŹĒ»·×“»ÆŗĻĪļC4H6O2 FµÄĢ¼Ō×ÓŌŚŅ»ĢõÖ±ĻßÉĻ
£Ø1£©»ÆŗĻĪļAŗ¬ÓŠµÄ¹ŁÄÜĶÅÓŠ ”£
£Ø2£©BŌŚĖįŠŌĢõ¼žĻĀÓėBr2·“Ó¦µĆµ½E£¬EŌŚ×ćĮæµÄĒāŃõ»ÆÄĘ“¼ČÜŅŗ×÷ÓĆĻĀ×Ŗ±äĪŖF£¬ÓÉE×Ŗ±äĪŖFŹ±·¢ÉśĮ½ÖÖ·“Ó¦£¬Ęä·“Ó¦ĄąŠĶ·Ö±šŹĒ ”£
£Ø3£©DµÄ½į¹¹¼ņŹ½ŹĒ ”£
£Ø4£©1mol AÓė2mol H2·“Ӧɜ³É1mol G£¬Ęä·“Ó¦·½³ĢŹ½ŹĒ ”£
£Ø5£©ÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĘŚÄ©Ģā ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com