
��ͼ��ʾ���ϳɰ�������ʾʵ��(�г���������ʡ��)����Y�ιܵ�һ����Zn����ϡH2SO4��Ӧ��ȡH2����һ����NaNO2�����NH4Cl������Һ��Ӧ��ȡN2��N2��H2��Ϻ�ͨ����ԭ�������ϳ�NH3���ٽ�����������ͨ���̪��Һ�У�����̪��Һ��죬��˵�������˰�����

ij����С��ͨ���������ϺͶ��ʵ�飬�õ���������Ϣ��
��Ϣһ��NaNO2����ͱ���NH4Cl��Һ��ϼ��ȵĹ����з������·�Ӧ��
��NaNO2��NH4Cl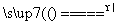 NH4NO2��NaCl
NH4NO2��NaCl
��NH4NO2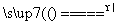 NH3����HNO2
NH3����HNO2
��2HNO2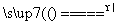 N2O3����H2O
N2O3����H2O
��2NH3��N2O3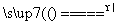 2N2��3H2O
2N2��3H2O
��Ϣ�����������ϣ���ͬ����ȵ�N2��H2�����������ͬʵ�������ºϳɰ���ʹ��̪��Һ�������Ҫ��ʱ�����£�
| N2��H2������� | 5��1 | 3��1 | 1��1 | 1��3 | 1��5 |
| ��̪���ɫ����ʱ��/min | 8��9 | 7��8 | 6��7 | 3��4 | 9��10 |
�ݴ˻ش��������⣺
(1)Y�ι������з�����Ӧ�����ӷ���ʽ________________________��
(2)��������ʯ�����ϵ�Ŀ����_________________________________ _______________________________________________________________________________________________________________��
(3)����С���ͬѧ����Ϊ����ʵ���м�ʹ��̪���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3���ó��˽��۵�������________________________��
���������һ����ʵ����֤�������____________________���������һ���⣬����ѡ����ͼ�е�________װ��������ԭװ���е�________��________֮�䡣
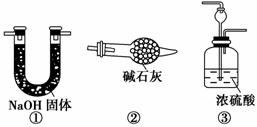
(4)������ʵ������У�Ϊ����۲쵽��̪��Һ����ʵ������Ӧ�ÿ���N2��H2�������Ϊ________�Ƚ����ˣ�����װ�û�����ʵ�ִ�Ŀ�ģ�ԭ����______________________________________��
(5)ʵ�������ͨ���Թ�C�е�����ɷ���________��
������(1)����Y�ι��Ҳ������ȣ�˵���Ҳ�ܷ�Ӧ��ȡN2�����ܷ�Ӧ��ȡH2��(2)��������ʯ�����ϵ�Ŀ����������������ĽӴ�������Ӷ���ߴ�Ч�ʣ�����Ӧ���ʡ�(3)��ΪNH4NO2�ֽ�ɲ���NH3�����Բ���֤��N2��H2��Ӧ������NH3��ֱ�ӽ�Y�ι��л������ͨ���̪��Һ������Һ��죬�����ɳ������������ɲ���������A��B֮���һ����NH3��װ�ã��ų���NH3�ĸ��š�(4)�ɱ���֪V(N2)��V(H2)��1��3ʱ����Ӧ��죬��Y�ι��У�����������������(5)����ɷ��������ɵ�NH3��ͬʱ����δ��Ӧ��N2��H2��
�𰸡�(1)Zn��2H��===Zn2����H2��
(2)����������������ĽӴ������ʹ��Ӧ���еø���
(3)�ӷֲ���Ӧ��֪������N2�Ĺ����У��п���ֱ�Ӳ�������������ϼ��Ȳ���������ֱ��ͨ���̪��Һ������Һ��죬��˵�����ɳ���������˵�����ɲ��������ۡ�A��B
(4)1��3��������ͨ��B��N2��H2�������
(5)NH3��N2��H2


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ױ���ú���ۺ����õõ��IJ���֮һ����ṹ��ʽΪ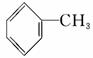 ���Իش��������⣺
���Իش��������⣺
(1)����ױ������ϵΪ________��
A��ͬ���칹�� B��ͬλ��
C��ͬ�������� D��ͬϵ��
(2)�ױ�ȼ��ʱ������Ϊ____________________________________________��
1 mol �ױ���ȫȼ���������������ʵ���Ϊ______________________��
(3)�ױ������ϵ�һ�ȴ�����________�֡�
(4)��֪���� �ṹ�����ʿɱ����Ը��������Һ���������ֱ��ͼױ��ķ�����_____________________________��
�ṹ�����ʿɱ����Ը��������Һ���������ֱ��ͼױ��ķ�����_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.100 0 mol��L��1 NaOH��Һ�ζ�20.00 mL 0.100 0 mol��L��1 CH3COOH��Һ���õζ���������ͼ������˵����ȷ���� (����)��
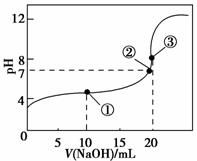
A�������ʾ��Һ�У�c(CH3COO��)��c(OH��)��c(CH3COOH)��c(H��)
B�������ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(CH3COO��)
C�������ʾ��Һ�У�c(Na��)>c(OH��)>c(CH3COO��)>c(H��)
D���ζ������п��ܳ��֣�c(CH3COOH)>c(CH3COO��)>c(H��)>c(Na��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ���ҵ�п��Ƶķ�Ӧ����Ӧ (����)��
A���¶�Խ��Խ��
B��ѹǿԽ��Խ��
C�������������������Խ��Խ��
D����ѡ�Ĵ�������Խ��Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڿ��淴ӦN2(g)��3H2(g)2NH3(g)����H<0������˵����ȷ����(����)��
A���ﵽƽ��ʱ��Ӧ���������Ũ��һ�����
B���ﵽƽ�����백�������´ﵽƽ��ʱ��������Ũ�ȱ�ԭƽ��ʱ��
C���ﵽƽ��ʱ�������¶ȼӿ������ȷ�Ӧ�����ʣ������˷��ȷ�Ӧ�����ʣ�����ƽ�����淴Ӧ�ķ����ƶ�
D����������������̵���ƽ���ʱ�䣬������Ϊ�ӿ�������Ӧ�����ʣ����������淴Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����
A. 20mL 10mol·L��1��Ũ�����Ũ����������ͭ���ȷ�Ӧת�Ƶ�������Ϊ0.2NA
B. 0.1mol�İ���(P4)������������Ĺ��ۼ�����Ϊ0.4NA
C. �ھ���ͭ����ͭ�Ĺ����У�����������ͭ32gת�Ƶ�������ΪNA
D. ��״���£�2.24L Cl2ͨ������H2O��NaOH��Һ��ת�Ƶĵ�������Ϊ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڸ���Һ����������ȷ����
A. pH��ȵĢ�NH4Cl����(NH4)2SO4����NH4HSO4����Һ�У�c(NH4��)��С���٣��ڣ���
B. �����£���10mL pH��12������������Һ�м���pH��2��HA��pH�պõ���7��������Һ���V(��)��20mL
C. ��1.00L 0.3mol·L��1��NaOH��Һ�л���ͨ��CO2��������Һ����8.8g��������Һ�У�3c(Na��)��2[c(HCO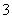 )��c(CO
)��c(CO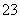 )��c(H2CO3)]
)��c(H2CO3)]
D. Ũ�Ⱦ�Ϊ0.1mol·L��1��CH3COOH��CH3COONa��Һ�������ϣ�
c(CH3COO��)��c(CH3COOH)��c(H��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ�м�������Na2O2�����ܴ���������������ǣ� ��
A��NH4+��Ba2+��Cl����NO3�� B��K+��AlO2����Cl����SO42��
C��Ca2+��Mg2+��NO3����HCO3�� D��Na+��Cl����CO32����SO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������1L pH=2�Ĵ�����Һ�м���2L pH=2�����ᣬ������Һ��pHΪ�������Ϻ���Һ������䣬�����´���ĵ���ƽ�ⳣ��Ϊ1.8��10-5���� ��
A��2.3 B��1.7 C��2 D����ȷ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com