
 ���б�Ȳ�������Ļ������5L�����������ڻ�������е��������Ϊx���ڴ��������£�����ַ�Ӧ��õ�����������ΪV L�������������ͬ��ͬѹ�²ⶨ����
���б�Ȳ�������Ļ������5L�����������ڻ�������е��������Ϊx���ڴ��������£�����ַ�Ӧ��õ�����������ΪV L�������������ͬ��ͬѹ�²ⶨ�������� ��Ȳ�ķ���ʽΪC3H4�������������ķ�Ӧ�У���C3H4+H2��C3H6��C3H6+H2��C3H8��C3H4+2H2��C3H8��
��V��C3H4����V��H2����1ʱ����Ӧ�����բٽ��У���V��C3H4����V��H2����$\frac{1}{2}$ʱ����Ӧ���۽��У���$\frac{1}{2}$��V��C3H4����V��H2����1ʱ�����ְ��ٽ��У����ְ��۷�Ӧ��
��V��C3H4����V��H2����$\frac{1}{2}$ʱ�����۷����ٻ�ۣ��õ��IJ�����C3H6����C3H8�����ǵĻ���ʣ�������������ڷ�Ӧ��C3H4�������
��V��C3H4����V��H2����$\frac{1}{2}$ʱ������������ʣ������Ϊ��C3H8��H2�Ļ����������ΪC3H4��ʣ��H2�Ļ���
��1����ǡ�÷�Ӧ����V��C3H4����V��H2��=1��2���ݴ˼���x��
��2�����������㣬��V��C3H4����V��H2����$\frac{1}{2}$ʱ����x��$\frac{2}{3}$���õ��IJ�����C3H6��C3H8�����ǵĻ���ʣ�������������ڷ�Ӧ��C3H4�������
��������������V��C3H4����V��H2����$\frac{1}{2}$ʱ����x��$\frac{2}{3}$������������ʣ��������������ΪC3H4��ʣ��H2�Ļ���
��3����x=$\frac{2}{3}$�������������ﵽ��Сֵ��x��$\frac{2}{3}$������x�����ӣ�����������V������x��$\frac{2}{3}$������x�����������V��С���ݴ˻���xΪ��ֵͬʱ��Ӧ������V��ͼ��
��� �⣺��Ȳ�ķ���ʽΪC3H4�������������ķ�Ӧ�У���C3H4+H2��C3H6��C3H6+H2��C3H8��C3H4+2H2��C3H8��
��V��C3H4����V��H2����1ʱ����Ӧ�����բٽ��У���V��C3H4����V��H2����$\frac{1}{2}$ʱ����Ӧ���۽��У���$\frac{1}{2}$��V��C3H4����V��H2����1ʱ�����ְ��ٽ��У����ְ��۷�Ӧ��
��V��C3H4����V��H2����$\frac{1}{2}$ʱ�����۷����ٻ�ۣ��õ��IJ�����C3H6����C3H8�����ǵĻ���ʣ�������������ڷ�Ӧ��C3H4�������
��V��C3H4����V��H2����$\frac{1}{2}$ʱ������������ʣ������Ϊ��C3H8��H2�Ļ����������ΪC3H4��ʣ��H2�Ļ���
��1����ǡ�÷�Ӧ�����ݷ�ӦC3H4+2H2��C3H8��֪��V��C3H4����V��H2��=1��2����x=$\frac{2}{3}$���ʴ�Ϊ��$\frac{2}{3}$��
��2����V��C3H4����V��H2����$\frac{1}{2}$ʱ����x��$\frac{2}{3}$������������ʣ��������������ΪC3H4��ʣ��H2�Ļ���V=V��C3H4��+Vʣ����H2��=5��1-x��L+5x-2��5��1-x��=10x-5��
���������㣬V��C3H4����V��H2����$\frac{1}{2}$ʱ����x��$\frac{2}{3}$���õ��IJ�����C3H6��C3H8����ߵĻ���ʣ�������������ڷ�Ӧ��C3H4�������V=V��C3H4��=5��1-x��L��
�ʴ�Ϊ��V=10x-5��V=5��1-x����
��3����x=$\frac{2}{3}$ʱ�������������ﵽ��Сֵ��x��$\frac{2}{3}$������x�����ӣ�����������V������x��$\frac{2}{3}$������x�����������V��С���ݴ˻���xΪ��ֵͬʱ��Ӧ������V��ͼ��Ϊ��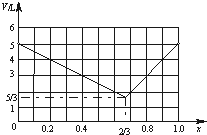 ��
��
��xΪ��ֵͬʱ��Ӧ������V��ͼ��Ϊ ��
��
���� ���⿼�����йط�Χ���۵Ļ�ѧ���㣬��Ŀ�ѶȽϴ���ȷ��д��Ӧԭ������Ӧ��Ӧ�ص�Ϊ�����Ĺؼ���ע�������йط�Χ���۵ļ��㷽��������������ѧ���ķ���������������


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������Ǵ������ܼӿ췴Ӧ���� | |
| B�� | �¶�Խ�ߣ������Ĵ�Ч��Խ�� | |
| C�� | ʳƷ��װ���еĿ����������ǡ�������������ʹ������ԭ��Ӧ�����ʼ��� | |
| D�� | �����¶ȣ����ȷ�Ӧ�ͷ��ȷ�Ӧ�ķ�Ӧ���ʶ��ӿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
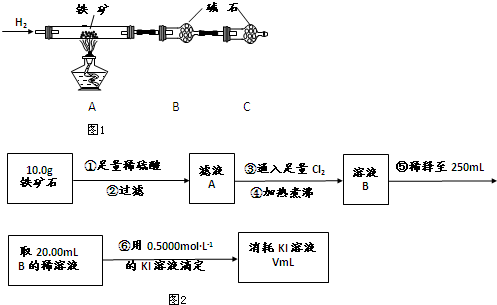
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 200 | B�� | 770 | C�� | 290 | D�� | 292 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���� | B�� | �춡�� | C�� | 2-��ϩ | D�� | 1-��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ϡHNO3 | B�� | CO | C�� | CH3CH2CH2OH | D�� | ϡH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�ش��� | A | B | D | E | G | H | I | J |
| ���ϼ� | -1 | -2 | +4��-4 | -1 | +5��-3 | +3 | +2 | +1 |
| ԭ�Ӱ뾶/nm | 0.071 | 0.074 | 0.077 | 0.099 | 0.110 | 0.143 | 0.160 | 0.186 |
| A�� | I��DB2��ȼ���������ֻ����� | |
| B�� | B��E��J�����Ӱ뾶�ɴ�С˳����E��J��B | |
| C�� | GԪ�صĵ��ʲ�����ͬ�������� | |
| D�� | B��J���γɼȺ����Ӽ��ֺ����ۼ��Ļ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
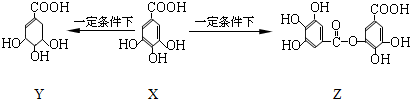
| A�� | 1 mol X�������2 mol Br2����ȡ����Ӧ | |
| B�� | Y���ӽṹ����3������̼ԭ�� | |
| C�� | Y�ܷ����ӳɡ�ȡ������ȥ������ | |
| D�� | 1 mol Z�������7 mol NaOH������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢� | D�� | �٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com