
|
�о�����ͭ������п��ϡ���ᷴӦ�����������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�飮�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䣮
����˵����ȷ���� | |
| [����] | |
A�� |
��Ӧһ��ʱ���ʵ��A��E�еĽ����ʰ���ɫ |
B�� |
V1��30��V6��10 |
C�� |
����MgSO4 ��Ag2SO4������������ͭ��ͬ�ļ������� |
D�� |
����ͭ����Խ�� ���������������ʿ϶�Խ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�� �����Һ |
A | B | C | D | E | F |
| 4mol/LH2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ �� �����Һ |
A | B | C | D | E | F |
| 4mol?L-1H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | 15 | 10 | 0 |
| ��� | c��HA��/mol?L-1 | c��NaOH��/mol?L-1 | ���ҺpH |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�¿��� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ�ĵ���У�����������¿���ѧ�Ծ� ���ͣ���ѡ��
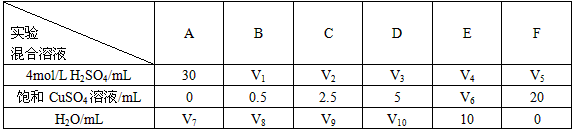
�о�����ͭ������п��ϡ���ᷴӦ�����������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�顣�����������Ļ����Һ�ֱ� ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol/L H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
���� CuSO4��Һ/mL CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com