
Ϊ�ⶨ�Ѳ��ֱ��ʵĹ���������Ʒ�Ĵ��ȣ��������ͼ��ʾ��ʵ��װ�ã�ͼ��QΪ�������õ�����ȡһ��������Ʒ�������У���ͼ��װ����������©���Ļ�������ϡ������������С�

����գ�
(1)Q�ڷ�����Ӧ����________�����壬������________��������ԭ��Ӧ��
(2)Ϊ�����Ӧʱ������������������ϡ����ǰ����ر�________(�K1����K2����K3������ͬ)����__________________��
(3)��������Ӧֹͣʱ����K1��K2��K3���ڹر�״̬��Ȼ���ȴ�K2���ٻ�����K1����ʱ�ɹ۲쵽��������_______________��
(4)b��װ�Ĺ����Լ���________��������K1������_______��
(5)ʵ�����ʱ����Ͳ������x mLˮ����Ͳ�����ռ�y mL���壬��������ƵĴ�����________(���������������ɱ�״��)��
���������ʵ�Na2O2�п��ܺ���̼���ƣ�����Ʒ��ϡ����Ӵ�������Ӧ��2Na2O2��2H2O===4NaOH��O2����H2SO4��2NaOH===Na2SO4��2H2O��H2SO4��Na2CO3===Na2SO4��CO2����H2O�����Q�ڲ���O2��CO2�������壬������һ����Ӧ����������ԭ��Ӧ���ⶨNa2O2�Ĵ��ȣ���ͨ��ֱ�ӲⶨCO2��O2������������������Ľ���������βⶨCO2��O2�����������ϡ�����������ǰ���ر�K1��K2����K3����Ӧ����ʱ�������͵��������Ͳ���������յ�ˮ�������ȣ���ΪCO2��O2�����֮�ͣ��������з�Ӧ��ȫ���ر�K3����K2���ٻ�����K1����ʱ�������徭�����b������Ͳ��CO2��������е����ռ����գ���Ͳ�����ų�ˮ�������ΪO2��������������֪��CO2��O2�����Ϊx mL��O2�����Ϊy mL����CO2�����Ϊ(x��y)mL����֪Na2O2��Na2CO3�����ʵ���֮��Ϊ2y(x��y)��Na2O2�Ĵ���Ϊw(Na2O2)��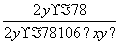 ��100%��
��100%��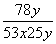 ��100%��
��100%��
�𰸡�(1)2��1��(2)K1��K2��K3��(3)����Q������С�����������Ͳ���С�(4)��ʯ�ҡ�������������٣�ʹCO2��������ա�(5)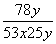 ��100%
��100%


 ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ��mA(g)+nB(g) pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������ ����Ҳ��С����:
����Ҳ��С����:
(1)�÷�Ӧ���淴ӦΪ_________�ȷ�Ӧ��m+n_________p(�������=��������)��
(2)��ѹʱ��A����������_________��(�������С�����䡱����ͬ)
(3)������B(�������)����A��ת����_________��B�ĵ�ת����_________��
 (4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮�� ��______ ___��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮�� ��______ ___��
(5)�����������ƽ��ʱ��������������ʵ���____ _____��
(6)��B����ɫ���ʣ�A��C����ɫ�������C(�������)ʱ�������ɫ______ _����ά��������ѹǿ���䣬��������ʱ���������ɫ____ ___(��������dz�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1�����(CH2Br—CH2—CH3)��2�����(CH 3—CHBr—CH3)�ֱ���NaOH���Ҵ���Һ���ȵķ�Ӧ�У�����Ӧ (����)
3—CHBr—CH3)�ֱ���NaOH���Ҵ���Һ���ȵķ�Ӧ�У�����Ӧ (����)
A��������ͬ����Ӧ������ͬ B�����ﲻͬ����Ӧ���Ͳ�ͬ
C��̼������ѵ�λ����ͬ D��̼������ѵ�λ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Fe��Cu��Fe2����Fe3����Cu2��ʢ��ͬһ�����г�ַ�Ӧ����Fe��ʣ�࣬��������ֻ����(����)
A��Cu��Fe3�� B��Fe2����Fe3��
C��Cu��Cu2����Fe D��Cu��Fe2����Fe
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ���Ͻ�10.2 g����������500 mL 4 mol·L��1���������Ҫʹ���������ﵽ���ֵ���������2 mol·L��1����������Һ�����Ϊ(����)
A��500 mL B��900 mL
C��1 000 mL D��1 200 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£���Ӧ2SO2(g)��O2(g)2SO3(g)���ﵽƽ��ʱ��n(SO2)��n(O2)��n(SO3)��2��3��4����С�������Ӧ�ٴδﵽƽ��ʱ��n(O2)��0.8 mol��n(SO3)��1.4 mol����ʱSO2�����ʵ���Ӧ��(����)
A��0.4 mol B��0.6 mol C��0.8 mol D��1.2 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��X������ͼ��ʾת����ϵ�����У�A��һ�����Σ�B����̬�⻯�C�ǵ��ʣ�F��ǿ�ᡣX������ǿ�ᣬҲ������ǿ�











(1)A�Ļ�ѧʽ��______________________________________��
(2)��X��ǿ�ᣬ��D��Cl2ͬʱͨ��ˮ�з�����Ӧ�����ӷ���ʽΪ________________________________��
(3)��X��ǿ�������B��Cl2��Ӧ������C�⣬��һ�������Ȼ��
�ٹ�����B��Cl2��Ӧ�Ļ�ѧ����ʽΪ________________________��
�ڹ�ҵ������B��D�Ļ�ѧ����ʽΪ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������[CH3CH(OH)COOH]��Ϊ���ǵ��о��ȵ�֮һ����������û�ѧ�����ϳɣ�Ҳ�����ɵ���ͨ������ͷ��Ʊ�������������й����⣺
(1)д��������������й����ŵ�����________��
(2) ��һ�������£��������ʲ��������ᷢ����Ӧ����________��
��һ�������£��������ʲ��������ᷢ����Ӧ����________��
A����ˮ B��NaOH��Һ
C��Cu(OH)2����Һ D��C2H5OH
(3)���Ա�ϩ(CH2===CH��CH3)Ϊ��Ҫԭ��(������ԭ����ѡ)�ϳ����ᣬ��ϳɹ��̵�����ͼ���£�

��Ӧ�ٵķ�Ӧ������________����Ӧ�ڵĻ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�����ڱ���Ԫ�������ɣ������Ʋ���ȷ���� (����)
A��H3BO3�����Ա�H2CO3��ǿ
B��Mg(OH)2�ļ��Ա�Be(OH)2��ǿ
C��HCl��HBr��HI�����ȶ���������ǿ
D����M����R2���ĺ�����Ӳ�ṹ��ͬ����ԭ��������R��M
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com