

 ���ڴ������ʵ������¿�������CPPO����
���ڴ������ʵ������¿�������CPPO����  �����Ƶ�
�����Ƶ� Ҳ���Է�����Ӧ����N����N�ṹ��ʽΪ_______________ ��д�����е�һ�֣���
Ҳ���Է�����Ӧ����N����N�ṹ��ʽΪ_______________ ��д�����е�һ�֣���
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
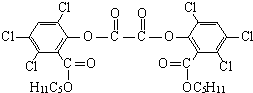 ��ħ�����������������յ���Ⱦ���䷢��ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ⣮�������
��ħ�����������������յ���Ⱦ���䷢��ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ⣮�������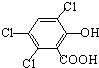 ���ڴ������ʵ������¿�������CPPO����
���ڴ������ʵ������¿�������CPPO���� ��
��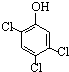 �루CH3��2C=CH2Ҳ���Է������Ƶķ�Ӧ����N����N�ṹ��ʽΪ
�루CH3��2C=CH2Ҳ���Է������Ƶķ�Ӧ����N����N�ṹ��ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��ͷ�г���һ�����ۻ�ѧ��ǰѺ��� ���ͣ�ʵ����
 ��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
�� ��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�
��﮻�ʯΪԭ���Ʊ�̼��﮵�һ���������£�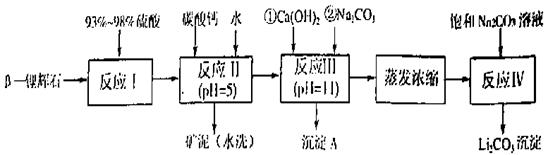
��֪���ٲ��ֽ����������↑ʼ��������ȫ������pH��
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 2.7 | 3.7 | 9.6 |
| ��ȫ����pH | 3.7 | 4.7 | 11 |
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
| A�������� | B�������� | C������̨������Ȧ�� | D���ƾ��� E��Բ����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ������ѧ�����п������ۻ�ѧ�Ծ��������棩 ���ͣ������
�ش��������⣨����ţ���
��1�����������У���©����������ƿ����������ƿ������ƽ���ݷ�Һ©��������Ͳ��
��ȼ�ճס����������ʷ������________________�����и������ʷе㲻ͬ���������ʵ�������____________�����������д��
��2��������NaOH��������220mL 0.2mol/L��NaOH��Һ������������գ�
A�����Ƹ���ҺӦѡ�ò�������_________ mL����ƿ��
B����������ƽ��ȡ ________ g NaOH���塣
C�����ƺõ�NaOH��������ձ��У�����Լ25mL����ˮ����__________��������ȫ�ܽ⡣����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
D������������ˮϴ���ձ��ڱںͲ�����2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ�� ����ҡ������ƿ��ʹ��Һ��;��ȡ�
E��������ƿ�м�������ˮ��ֱ��Һ����̶�����1-2����ʱ������___________ �μ�����ˮ��Һ����̶������С��Ǻ�ƿ����ҡ�ȡ������Һδ��ȴ�ͽ��ж��ݣ���ʹ��õ���ҺŨ��___________���ƫ�ߡ�����ƫ�͡��� ����Ӱ�족����
F�����ƺõ���Һ__________����ܡ����ܡ������ڴ��������ƿ�С�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com