
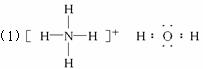
��1�����A��B��C��D����10���ӵ����ӣ���д����
A�Ľṹʽ____________________��D�ĵ���ʽ____________________��
��2�����A��C��18���ӵ����ӣ�B��D��10���ӵ����ӣ���д����
��A��B����Һ�з�Ӧ�����ӷ���ʽ��____________________��
�ڸ����������ӷ���ʽ�����ж�C��B������ӵ�������С�ǣ��û�ѧʽ�����ӷ��ţ���ʾ��____________________��
��3����֪�£�H2N��NH2���ͼװ���CH3��NH2�����Ǻ�18�����ӵķ��ӡ������ºͼװ��Ľṹ�ص㲢�����ܵ�������д����������ͬ���������л�������Ľṹ��ʽ��������д������
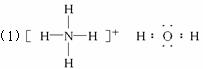
��2����H2S+OH-====HS-+H2O(��H2S+2OH-====S2-+2H2O��
��OH-ǿ��HS-����S2-��
��3��CH3��CH3 CH3��OH CH3��F
��������10��������Na+��Mg2+��Al3+��![]() ��H3O+��N3-��O2-��F-��OH-��
��H3O+��N3-��O2-��F-��OH-��![]() ��Ne��HF��H2O��NH3��CH4�����ҳ�����ͼʾ�����ӣ�AΪ
��Ne��HF��H2O��NH3��CH4�����ҳ�����ͼʾ�����ӣ�AΪ![]() ��BΪOH-��CΪNH3��DΪH2O��18����������K+��Ca2+��P3-��S2-��HS-��Cl-��Ar��HCl��H2S��PH3��SiH4��F2��H2O2��C2H6��CH3OH��CH3F��N2H4�ȣ���ͼʾ��A��C��18�������ӣ�B��D��10�������ӣ���AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��C2H6��CH3OH��CH3F�Ⱦ�Ϊ�л��
��BΪOH-��CΪNH3��DΪH2O��18����������K+��Ca2+��P3-��S2-��HS-��Cl-��Ar��HCl��H2S��PH3��SiH4��F2��H2O2��C2H6��CH3OH��CH3F��N2H4�ȣ���ͼʾ��A��C��18�������ӣ�B��D��10�������ӣ���AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��C2H6��CH3OH��CH3F�Ⱦ�Ϊ�л��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
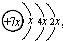 ��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ�����
��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
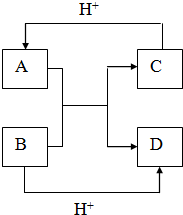 ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��A��ԭ�Ӱ뾶��B��С | B��B��C�γɵĻ�����ֻ��һ�� | C��C�ڻ������г�+1�� | D��D���ʵľ���������뵼����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com