
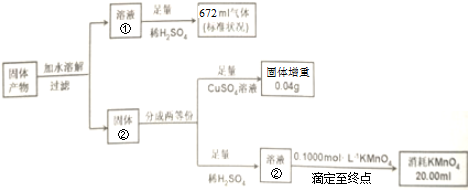
·ÖĪö AŌŖĖŲµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹż2±¶£¬AĪŖCŌŖĖŲ£¬BŌŖĖŲµÄÖ÷×åŠņŹżŹĒĘäĖłŌŚÖÜĘŚŹżµÄ3±¶£¬BĪŖOŌŖĖŲ£®¼×”¢ŅŅ¾łÓÉC”¢OĮ½ŌŖĖŲ×é³É£¬ĒŅĦ¶ūÖŹĮæ£ŗM£Ø¼×£©£¼M£ØŅŅ£©£¬¹Ź¼×ĪŖCO£¬ŅŅĪŖCO2£®»ÆŗĻĪļK3Fe£ØC2O4£©3•3H2OŹÜČČĶźČ«·Ö½ā·½³ĢŹ½ĪŖ2K3Fe£ØC2O4£©3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O£®¹ĢĢå²śĪļÖ»ÓŠFe”¢FeOŗĶK2CO3£¬¼ÓĖ®ČܽāŗóČÜŅŗ¢ŁĪŖĢ¼Ėį¼Ų£¬¹ĢĢåĪŖFe”¢FeO£¬ĢśæÉÓėĮņĖįĶČÜŅŗ·“Ӧɜ³ÉĶµ„ÖŹ£¬Fe”¢FeO¶¼æÉÓėĮņĖį·“Ó¦£¬·“Ӧɜ³ÉµÄ¶ž¼ŪĢśæÉÓĆKMnO4ČÜŅŗµĪ¶Ø£®
½ā“š ½ā£ŗAŌŖĖŲµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹż2±¶£¬AĪŖCŌŖĖŲ£¬BŌŖĖŲµÄÖ÷×åŠņŹżŹĒĘäĖłŌŚÖÜĘŚŹżµÄ3±¶£¬BĪŖOŌŖĖŲ£®¼×”¢ŅŅ¾łÓÉC”¢OĮ½ŌŖĖŲ×é³É£¬ĒŅĦ¶ūÖŹĮæ£ŗM£Ø¼×£©£¼M£ØŅŅ£©£¬¹Ź¼×ĪŖCO£¬ŅŅĪŖCO2£®»ÆŗĻĪļK3Fe£ØC2O4£©3•3H2OŹÜČČĶźČ«·Ö½ā·½³ĢŹ½ĪŖ2K3Fe£ØC2O4£©3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O£®¹ĢĢå²śĪļÖ»ÓŠFe”¢FeOŗĶK2CO3£¬¼ÓĖ®ČܽāŗóČÜŅŗ¢ŁĪŖĢ¼Ėį¼Ų£¬¹ĢĢåĪŖFe”¢FeO£¬ĢśæÉÓėĮņĖįĶČÜŅŗ·“Ӧɜ³ÉĶµ„ÖŹ£¬Fe”¢FeO¶¼æÉÓėĮņĖį·“Ó¦£¬·“Ӧɜ³ÉµÄĮņĖįŃĒĢśÄÜŹ¹KMnO4ČÜŅŗĶŹÉ«£®
£Ø1£©¼×µÄ»ÆѧŹ½£ŗCO£¬¹Ź“š°øĪŖ£ŗCO£»
£Ø2£©ČÜŅŗ¢ŚĪŖĮņĖįŃĒĢś£¬ÓėKMnO4·¢ÉśŃõ»Æ»¹ŌµÄĄė×Ó·½³ĢŹ½£ŗMnO4-+5Fe2++8H+ØTMn2++5Fe3++4H2O£¬¹Ź“š°øĪŖ£ŗMnO4-+5Fe2++8H+ØTMn2++5Fe3++4H2O£»
£Ø3£©»ÆŗĻĪļK3Fe£ØC2O4£©3•3H2OŹÜČČĶźČ«·Ö½ā·½³ĢŹ½ĪŖ2K3Fe£ØC2O4£©3•3H2O=3K2CO3+Fe+FeO+5CO2+4CO+6H2O£¬¹Ź“š°øĪŖ£ŗ1£»1£»3£»
£Ø4£©øßĆĢĖį¼ŲČÜŅŗ³Ź×ĻÉ«£¬ĮņĖįŃĒĢśÓėøßĆĢĖį¼ŲČÜŅŗĒ”ŗĆ·“Ó¦Ź±£¬ČÜŅŗŹĒĪŽÉ«µÄ£¬µ±øßĆĢĖį¼Ų¹żĮæŹ±ČÜŅŗ³Ź×ĻÉ«£¬ÓĆøßĆĢĖį¼ŲČÜŅŗµĪ¶ØĮņĖįŃĒĢśČÜŅŗÖĮÖÕµć£ŗµĪČė×īŗóŅ»µĪøßĆĢĖį¼ŲČÜŅŗ£¬ČÜŅŗĒ”ŗĆÓÉĪŽÉ«±äĪŖ×ĻÉ«£¬¹Ź“š°øĪŖ£ŗ²»ŗĻĄķ£¬Ó¦ĪŖµĪČė×īŗóŅ»µĪøßĆĢĖį¼ŲČÜŅŗ£¬ČÜŅŗĒ”ŗĆÓÉĪŽÉ«±äĪŖ×ĻÉ«£®
µćĘĄ ±¾Ģāæ¼²éĢ½¾æĪļÖŹµÄ×é³É»ņ²āĮæĪļÖŹµÄŗ¬Į棬ĪŖøßĘµæ¼µć£¬°ŃĪÕĪļÖŹµÄĶʶĻĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲŃõ»Æ»¹Ō·“Ó¦ÅäĘ½¼°»ł±¾øÅÄīµÄ漲飬ĢāÄæÄѶČÖŠµČ£®


 Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø
Š”ѧæĪĢĆ×÷ŅµĻµĮŠ“š°ø ½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
½š²©ŹæŅ»µćČ«ĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś | B£® | ¢Ś¢Ū | C£® | ¢Ł¢Ū | D£® | ¢Ś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¾ŪŅŅĻ©ĖÜĮĻµÄĄĻ»ÆŹĒÓÉÓŚ·¢ÉśĮĖ¼Ó³É·“Ó¦ | |
| B£® | Ćŗ¾¹żĘų»ÆŗĶŅŗ»ÆµČĪļĄķ±ä»ÆæÉŅŌ×Ŗ»ÆĪŖĒå½ąČ¼ĮĻ | |
| C£® | ĢśŌŚ³±ŹŖµÄæÕĘųÖŠ·ÅÖĆ£¬Ņ×·¢Éś»ÆѧøÆŹ“¶ųÉśŠā | |
| D£® | »ŖŅįæĘѧ¼ŅøßļæŌŚ¹āĻĖ“«ŹäŠÅĻ¢ĮģÓņÖŠČ”µĆĶ»ĘĘŠŌ³É¾Ķ£¬¹āĻĖµÄÖ÷ŅŖ³É·ÖŹĒøß“æ¶ČµÄ¶žŃõ»Æ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀĻĀ£¬·“Ó¦2A £Øs£©+B £Øg£©ØT2C £Øg£©+D £Øg£©²»ÄÜ×Ō·¢½ųŠŠ£¬ŌņøĆ·“Ó¦”÷HŅ»¶Ø“óÓŚ0 | |
| B£® | 101kPaŹ±£¬2 H2£Øg£©+O2£Øg£©ØT2H2O£Øl£©”÷H=-571.6kJ•mol-1£¬ŌņH2µÄČ¼ÉÕČČĪŖ571.6kJ•mol-1 | |
| C£® | C £ØŹÆÄ«£¬s£©ØTC £Ø½šøÕŹÆ£¬s£©”÷H1=+1.9kJ•mol-1£¬ŌņÓÉŹÆÄ«ÖĘČ”½šøÕŹÆµÄĪüČČ·“Ó¦£¬½šøÕŹÆ±ČŹÆÄ«ĪČ¶Ø | |
| D£® | 0.5molH2SO4Óė0.5mol Ba£ØOH£©2ĶźČ«·“Ó¦Ėł·Å³öµÄČČĮ漓ĪŖÖŠŗĶČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ü | B£® | ¢Ś¢Ū¢Ž | C£® | ¢Ü¢Ż¢Ž | D£® | ¢Ū¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Al3+µÄ½į¹¹Ź¾ŅāĶ¼£ŗ | B£® | Ė®µÄµē×ÓŹ½£ŗ | ||
| C£® | ŅŅĻ©µÄ½į¹¹¼ņŹ½£ŗCH2CH2 | D£® | ŗ¬ÓŠ7øöÖŠ×ÓµÄĢ¼Ō×Ó£ŗ${\;}_{6}^{7}$C |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| Ö÷×å ÖÜĘŚ | ¢ńA | ¢ņA | ¢óA | ¢ōA | ¢õA | ¢öA | ¢÷A | 0×å |
| 2 | ¢Ł | ¢Ś | ¢Ū | |||||
| 3 | ¢Ü | ¢Ż | ¢Ž | ¢ß | ¢ą |
 £¬½«¹żĮæAĶØČėÓÉ¢ŚŗĶ¢ŻŠĪ³ÉµÄŅõĄė×ÓµÄŃĪČÜŅŗµÄĄė×Ó·½³ĢŹ½ĪŖAlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£®
£¬½«¹żĮæAĶØČėÓÉ¢ŚŗĶ¢ŻŠĪ³ÉµÄŅõĄė×ÓµÄŃĪČÜŅŗµÄĄė×Ó·½³ĢŹ½ĪŖAlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N2 | B£® | SO2 | C£® | CO2 | D£® | CO |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com