
��11�֣�
��ͬѧ��������װ����֤ľ̿��Ũ���ᷴӦ��ȫ������
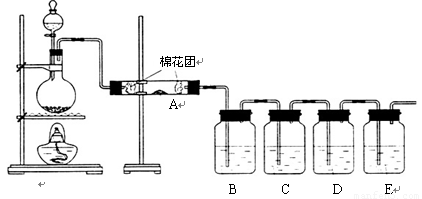
��1��д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2��A�м�����Լ��� ��B��D�м�����Լ�����Ʒ����Һ��D����ȷ��ʵ�������ǣ�
��3��ʵ��ʱ��C����������ǵ��е��۵ĵ�ˮ���۲쵽�������� ��
���ӷ���ʽΪ�� ��
����ͬѧֻ��B��C��D��Eװ����֤SO2��ijЩ���ʣ���ش��������⣺
��1��C�м�����Լ��� ��֤��SO2���������ԡ�
��2��D�м������Ե�KMnO4��Һ��֤��SO2���� �ԡ�
��3��E�м�����з�̪��NaOH��Һ��֤��SO2�� �����塣

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
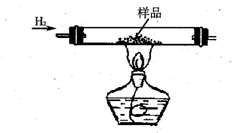 ʵ������CuO��ͭ�۵Ļ���Ҫ�ⶨ����Ʒ���ѳƵ�������Ϊm g��������ͭ������������
ʵ������CuO��ͭ�۵Ļ���Ҫ�ⶨ����Ʒ���ѳƵ�������Ϊm g��������ͭ������������| ʵ�鷽�� | �йػ�ѧ����ʽ | ʵ��������������ţ� | ʵ����ֱ�Ӳⶨ����������������˵���� |
| ��1�� | H2SO4+CuO�TCuSO4+H2O | ||
| ��2�� | ʵ��ǰ��������Ʒ����������������������ʵ���������CuO������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11�֣�
��ͬѧ��������װ����֤ľ̿��Ũ���ᷴӦ��ȫ������
��1��д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2��A�м�����Լ��� ��B��D�м�����Լ�����Ʒ����Һ��D����ȷ��ʵ�������ǣ�
��3��ʵ��ʱ��C����������ǵ��е��۵ĵ�ˮ���۲쵽�������� ��
���ӷ���ʽΪ�� ��
����ͬѧֻ��B��C��D��Eװ����֤SO2��ijЩ���ʣ���ش��������⣺
��1��C�м�����Լ��� ��֤��SO2���������ԡ�
��2��D�м������Ե�KMnO4��Һ��֤��SO2���� �ԡ�
��3��E�м�����з�̪��NaOH��Һ��֤��SO2�� �����塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡüɽ��ѧ��һ��ѧ����ĩ��ѧ������⻯ѧ���� ���ͣ�ʵ����
��11�֣�
��ͬѧ��������װ����֤ľ̿��Ũ���ᷴӦ��ȫ������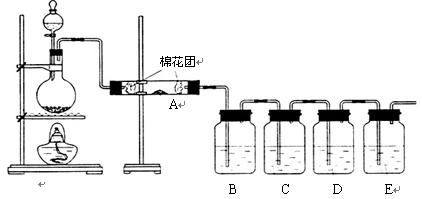
��1��д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2��A�м�����Լ��� ��B��D�м�����Լ�����Ʒ����Һ��D����ȷ��ʵ�������ǣ�
��3��ʵ��ʱ��C����������ǵ��е��۵ĵ�ˮ���۲쵽�������� ��
���ӷ���ʽΪ�� ��
����ͬѧֻ��B��C��D��Eװ����֤SO2��ijЩ���ʣ���ش��������⣺
��1��C�м�����Լ��� ��֤��SO2���������ԡ�
��2��D�м������Ե�KMnO4��Һ��֤��SO2���� �ԡ�
��3��E�м�����з�̪��NaOH��Һ��֤��SO2�� �����塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬѧ��������װ����֤ľ̿��Ũ���ᷴӦ��ȫ������
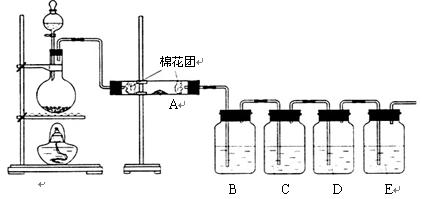
��1��д��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��2��A�м�����Լ��� ��B��D�м�����Լ�����Ʒ����Һ��D����ȷ��ʵ�������ǣ�
��3��ʵ��ʱ��C����������ǵ��е��۵ĵ�ˮ���۲쵽�������� ��
���ӷ���ʽΪ�� ��
����ͬѧֻ��B��C��D��Eװ����֤SO2��ijЩ���ʣ���ش��������⣺
��1��C�м�����Լ��� ��֤��SO2���������ԡ�
��2��D�м������Ե�KMnO4��Һ��֤��SO2���� �ԡ�
��3��E�м�����з�̪��NaOH��Һ��֤��SO2�� �����塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com