
ij�л���A������������ܶ���38��ȡ�л���A 7.6 g��ȫȼ�պ�����0.2 mol CO2��0.2 mol H2O�����л���ȿ�������Ʒ�Ӧ���ֿ����������ƺ�̼���Ʒ�Ӧ��
�����й�A��˵���в���ȷ���ǣ� ��
A��A�ķ���ʽΪC2H4O3
B��A�Ľṹ��ʽΪHO��CH2��COOH
C��A�����еĹ�����������
D��1 mol A�������ĵ���Na��Ӧʱ�ų�H2�����ʵ���Ϊ0.5 mol
D
��������
����������л���A������������ܶ���38����A����Է���������76������7.6g�л�������ʵ�����0.1mol�������ԭ���غ��֪��A�к��е�̼����ԭ�Ӹ����ֱ���2��4.���Ժ��е���ԭ�Ӹ�����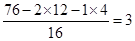 ��������ʽΪC2H4O3�����л���ȿ�������Ʒ�Ӧ���ֿ����������ƺ�̼���Ʒ�Ӧ�����Է��Ӻ����Ȼ����ǻ������ѡ��D����ȷ��Ӧ����1mol����������ѡ�����ȷ�ģ���ѡD��
��������ʽΪC2H4O3�����л���ȿ�������Ʒ�Ӧ���ֿ����������ƺ�̼���Ʒ�Ӧ�����Է��Ӻ����Ȼ����ǻ������ѡ��D����ȷ��Ӧ����1mol����������ѡ�����ȷ�ģ���ѡD��
���㣺�����л������ʽ���ṹ��ʽ���йؼ�����ж�
�����������л���ķ���ʽ�ǣ��ؼ������ø����غ㷨���У��ر����ܷ�ȷ�жϳ��Ƿ���ԭ�ӡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡâ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� �� ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡâ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0108 ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������ѧ�߶����ϣ����л�ѧ�Ծ���ѡ�ޣ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com