
 CuO + SO3��
CuO + SO3�� 2
2 SO2��+ O2��
SO2��+ O2��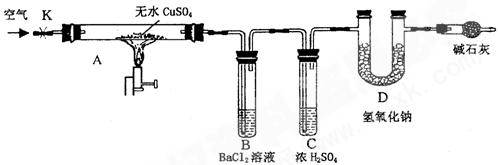
 �ܵ�������
�ܵ������� ��
�� ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| | �ŵ� | ȱ�� |
| ��װ�� | | |
| ��װ�� | | |
| ��װ�� | | |
�鿴�𰸺ͽ���>>
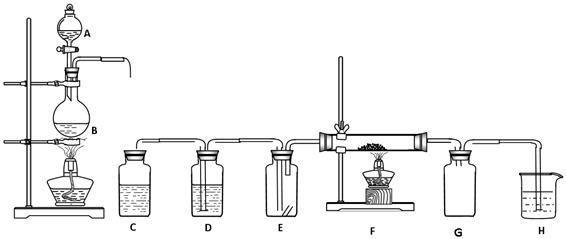
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ζ�ǰ��ƿ������ˮ��ϴ�� |
| B���ζ�ǰ��ƿ�м������������ּ�����ˮ |
| C���ζ�ǰ����������ȷ���ζ����Ӷ��� |
| D���ζ�ǰ����������δ�ų������еζ����ζ������������ |
�鿴�𰸺ͽ���>>
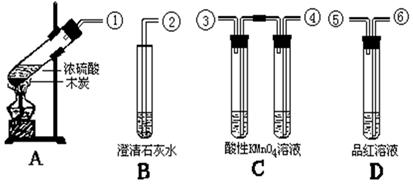
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����SO2ͨ��BaCl2��Һ�в�����ɫ�������ٵμ�ϡ�����������ʧ |
| B���ñ�����������Һ�ζ�һ���������ȵ����ᣬ�յ�ʱ��Һ�ɳ�ɫ��ɻ�ɫ |
| C��ȡ�����ӵ�ʳ�μ��������Һ�У���ֽ������Һ����ɫ |
| D������FeSO4��Һʱ����������ϡ������������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
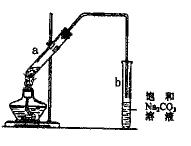
| A����a�Թ����ȼ���Ũ���ᣬȻ���ҡ���Թܱ����������Ҵ����ټ����� |
| B���Թ�b�е������¶˹ܿڲ��ܽ���Һ���Ŀ���Ƿ�ֹʵ������в����������� |
| C���Ƶõ�����������һ����ɫ��������ζ����״Һ�� |
| D����a�Թ��м��뼸�����Ƭ�������Ƿ�ֹ����ʱҺ�屩�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com