
��15�֣���ͼ��ʾװ�ÿ�������ȡFe(OH)2�۲�Fe(OH)2�ڿ����б�����ʱ����ɫ�仯��ʵ��ʱ����ʹ����м��6 mol/L������Һ�������Լ���ѡ����д���пհף�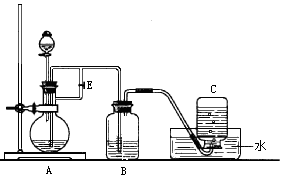
��1��B��ʢ��һ������NaOH��Һ��D���������� ����ʵ��D��������Ҫ
���� ��Һ��A��ӦԤ�ȼ�����Լ��� ��A�з�Ӧ�����ӷ���ʽ
Ϊ ��
��2��ʵ�鿪ʼʱӦ�Ƚ�����E ������رա����� ��C���յ���������ҪΪ ����Cƿ��Aƿ�е����� ʱ��������E �����
�رա������˿�Bƿ�п��ܷ����Ļ�ѧ��Ӧ�������ӷ���ʽ��ʾ���ǣ�
_ ��
��3����ȥװ��B�е���Ƥ����ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ��
��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����22�֣�
��4�֣�����ʵ������У���������
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
��10�֣�ʵ��������ͼ��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��
(1) �ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
(2) D�Թ���װ���� ���������� ��
(3) E�Թ���װ���� ��E�Թ��ڳ��ֵ�����Ϊ ��
(4) ��������ƿ�еķ�Ӧ��������ʱ(��ʱ�������Լ���)����F�������ر�C���������Կ����������� ��
(5) ��һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
��8�֣�������ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1�� ��BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��________________ ��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е����� ��
��2�� ��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ�.��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E.Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A�� �������ƣ���C�� ���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ�� _____________������D�ڴ�ʵ���е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
����22�֣�
��4�֣�����ʵ������У��������� 
| A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170�� |
| B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ |
| C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ�� |
| D�������еμ�����ϡ��ˮ���������������鱽�� |

 ϴ
ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�����д���пհף�
��1��װ�â�����������װ�ã�Բ����ƿ��ʢ��MnO2���壬�䷴Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ʵ������MnO2�����ˣ����������ʿ��ܿ�����������MnO2��Cl2���ǣ�
A��NaBiO3 B��FeCl3 C��PbO2
����֪������ǿ��˳��Ϊ��NaBiO3��PbO2��MnO2��FeCl3��
��3���Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����Ǣ� �� �� ��
��4����Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ��� (��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ����� ����дʵ��������ƣ���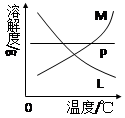
��5��һ������������������ȼ�գ����õĻ������100mL 3.00mol/L��NaOH��Һ���ܶ�Ϊ1.2g/mL��ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.0500mol��������Һ��Cl�����ӵ����ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�

ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�����д���пհף�
��1��װ�â�����������װ�ã�Բ����ƿ��ʢ��MnO2���壬�䷴Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ʵ������MnO2�����ˣ����������ʿ��ܿ�����������MnO2��Cl2���ǣ�
A��NaBiO3 B��FeCl3 C��PbO2
����֪������ǿ��˳��Ϊ��NaBiO3��PbO2��MnO2��FeCl3��
��3���Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����Ǣ� �� �� ��
��4����Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ��� (��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ����� ����дʵ��������ƣ���

��5��һ������������������ȼ�գ����õĻ������100mL 3.00mol/L��NaOH��Һ���ܶ�Ϊ1.2g/mL��ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.0500mol��������Һ��Cl�����ӵ����ʵ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com