
ʵ����������������ҩƷ��ȡ���ռ����﴿����������

(1)�����������ң�װ����ȷ�������ǣ�a�ӣ� ������ ���ӣ� ������ ���ӣ� ������ ���ӣ� ��������ӿ���ĸ��
��2��װ��F�������� ��װ��E�������� ��
��3��װ��A��E�еķ�Ӧ��ѧ����ʽΪ��A ��
E ��
��4����A����4.48L��������(��״̬��)����μӷ�Ӧ��HCl
�� g��
��5����������������ͨ��KI������Һ���ܹ۲쵽 ����Ӧ�����ӷ���ʽΪ ��
 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
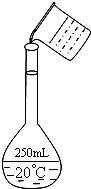 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ��500mL����ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ��500mL����ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com