
̼���γɻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʡ���ش��������⣺
��1���л���M����̫������տ�ת����N��ת���������£�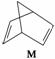
 ����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
��2����֪CH3OH(l)��ȼ���� Ϊ238.6 kJ·mol��1��CH3OH(l)��
Ϊ238.6 kJ·mol��1��CH3OH(l)��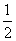 O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
��3��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��______________________________��
��4������͵�������ı������������ʡ���ʯī�����ۺͶ������Ѱ�һ����������ڸ��������գ��������ʿ������²��ϣ�4Al(s)��3TiO2(s)��3C(s)===2Al2O3(s)��3TiC(s)����H����1 176 kJ·mol��1����Ӧ�����У�ÿת��1 mol���ӷų�������Ϊ____________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ú����ˮ����Na2S2O3��5H2O��ʵ���ҿ�������װ��(��ȥ���ּг�����)ģ���������̡�
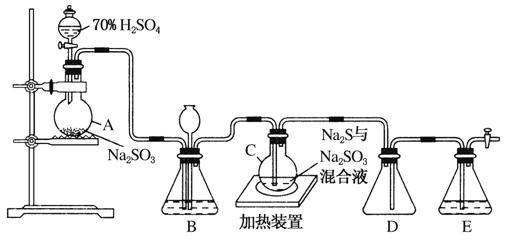
��ƿC�з�����Ӧ���£�
Na2S(aq)��H2O(l)��SO2(g)===Na2SO3(aq)��H2S(aq)��(��)
2H2S(aq)��SO2(g)===3S(s)��2H2O(l)��(��)
S(s)��Na2SO3(aq)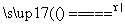 Na2S2O3(aq)��(��)
Na2S2O3(aq)��(��)
(1)������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������______________________��������װ�����������á�װ��D��������________��װ��E��Ϊ________��Һ��
(2)Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ________��
(3)װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��________(��ѡ����ĸ����ͬ)��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ�����___________��
��֪��Ӧ(��)��Խ���������ƿC�з�Ӧ�ﵽ�յ��������______����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������________��
a���ձ� b��������
c���Թ� d����ƿ
(4)��Ӧ��ֹ����ƿC�е���Һ������Ũ������ȴ�ᾧ������Na2S2O3��5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ���
__________________________________________________________��
��֪Na2S2O3��5H2O�����ֽ⣺S2O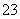 ��2H��===S����SO2����H2O
��2H��===S����SO2����H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������Թ�ҵ��ˮ�к���һ������Fe3����Cu2����Au3�������ӡ����������ͼ�еĹ������̣����ó��õ��ᡢ���ҵ�����еķ���м���ӷ�ˮ�л��ս𣬲�����һ���������������ͭ��
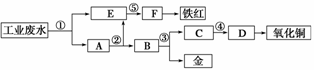
��д����հף�
(1)ͼ�б�Ŵ���������Ӧ���ʷֱ���
��____________����____________����____________��
��____________����____________��
(2)д���ٴ�������Ӧ�����ӷ���ʽ
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
д���۴�������Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________��
(3)����Ļ�ѧʽΪ________���ֱ�д�����������ͭ�ڹ�ҵ�ϵ�һ����Ҫ��;��
����___________________________________________________________________��
����ͭ_________________________________________________________________��
������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�߶���ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ�������¡��Ը����±��е�ʵ������ش��������⣺
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
(1)ʵ��1��2��Al�����ĵ缫�Ƿ���ͬ��________(��ǡ���)��
(2)ʵ��3�еĵ缫��Ӧʽ������______________________������______________________________������ܷ�Ӧ����ʽ______________________��
(3)ʵ��4��Al��________������缫��Ӧʽ��____________________________���жϵ缫��������_____________________________________________________________��
(4)����ʵ��5�е�����ָ��ƫ��Al��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ������Ҫ�� ���Ǽ��飬������һ�����ʵ�ȼ�ϣ���������Ļ���ԭ�ϡ����ⷴӦ��ͨ������������
���Ǽ��飬������һ�����ʵ�ȼ�ϣ���������Ļ���ԭ�ϡ����ⷴӦ��ͨ������������
Ӧʵ�֣���CH4(g)��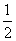 O2(g)===CO(g)��2H2(g)����H����36 kJ/mol����CH4(g)��H2O(g)===CO(g)��3H2(g)����H
O2(g)===CO(g)��2H2(g)����H����36 kJ/mol����CH4(g)��H2O(g)===CO(g)��3H2(g)����H
����216 kJ/mol������˵����ȷ��������
A�������������Ȼ�ѧ����ʽ���ܵó�������������Ӧ������̬ˮ���Ȼ�ѧ����ʽ
B����Ӧ�ٲ����Է�����
C���������һ�ֻ�ʯ��Դ������һ�ֿ�������Դ
D��������������Ӧ���Լ����������ȼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH4��H2��CO��ȼ���ȷֱ�Ϊ890.31kJ/mol��285.8kJ/mol��110.5 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ����
A��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=-890.31kJ/mol
B��2H2(g)+ O2(g)= 2H2O(l) ��H=-285.8kJ/mol
 C��CO (g)+ H2O(g)= CO2(g)+
C��CO (g)+ H2O(g)= CO2(g)+  H2 (g) ��H=+175.3kJ/mol
H2 (g) ��H=+175.3kJ/mol
D��2CO (g)+ O2(g) = 2CO2(g) ��H=-221 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���1mol��CuSO4·5H2O(s)����ˮ��ʹ��Һ�¶Ƚ��ͣ���ЧӦΪ��H1����1mol��CuSO4(s)����ˮ��ʹ��Һ�¶����ߣ���ЧӦΪ��H2��CuSO4·5H2O���ȷֽ�Ļ�ѧ����ʽΪ��CuSO4·5H2O(s) 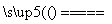 CuSO4(s)+5H2O(l)�� ��ЧӦΪ��H3���������ж���ȷ����
CuSO4(s)+5H2O(l)�� ��ЧӦΪ��H3���������ж���ȷ����
A����H2����H3  B����H1����H3
B����H1����H3
C����H1+��H3 =��H2  D����H1+��H2 ����H3
D����H1+��H2 ����H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ũ��Ϊ0.1 mol��L��1��K2CO3��Һ�У��ֱ�����������ʣ���ʹ[CO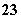 ]�������(����)
]�������(����)
��H2O ��CO2 ��K2S ��KOH
A���٢� B���٢� C���ڢ� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C�������ڱ��ж�����Ԫ����ɵ����ֳ���������ס��ҡ��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��ʾ�Ĺ�ϵ��
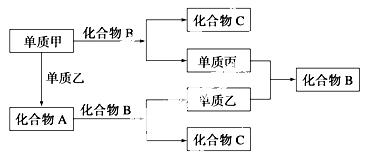
������пհף�
��1��A��B��C�Ļ�ѧʽ�ֱ�Ϊ________��______��______��
��2�����ʼ��뻯����B��Ӧ�����ӷ��� ʽΪ________________��
ʽΪ________________��
��3����A��B��C���ֻ������У��ض����е�Ԫ����________(��Ԫ�ط��ű�ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com