
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g) 2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
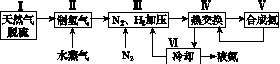
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
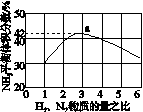
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
(1)2NH4HS��O2 2NH3��H2O��2S��
2NH3��H2O��2S��
(2)a��90%
(3)14.5%
(4)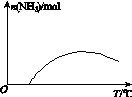
(5)������ԭ������ѹ������Һ����δ��Ӧ��N2��H2ѭ��ʹ��
[����] (1)�������֪Ϊ�����е�O2��������������Ϊ���ʣ����ݵ����غ㽫����ʽ��ƽ���ɡ�(2)��Ӧ��Ϊ�������ʵ�����������ȷ�Ӧ������ѹǿʹƽ�����ƣ�����Ӧ���ʼ�С��d�����������ܸı䷴Ӧ�ȣ������ܸı�H2�İٷֺ�����c��������ˮ����Ũ�����ʹ��Ӧ���������Լ�ƽ�����ƣ�������H2�İٷֺ���ȴ��С��b���������¶ȷ�Ӧ����������ƽ�������ƶ���H2�İٷֺ�������a�ԡ�CO��H2�Ļ��������ˮ�����ķ�Ӧ�У���Ӧ��ϵ�е���������ʵ������䣬��1 molCO��H2�Ļ������μӷ�Ӧ����1.18 mol�������˵����0.18 mol ˮ�����μӷ�Ӧ������ݷ���ʽ�ڿ�֪�μӷ�Ӧ��COҲΪ0.18 mol������ת����Ϊ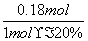 ��100%��90%��
��100%��90%��
(3)��ͼ�п�����N2��H2���ʵ�����Ϊ1��3ʱ��NH3��ƽ������������Ϊ42%����ƽ��ʱת����N2�����ʵ���Ϊx mol��������ʽ��
����������N2��3H2 2NH3
2NH3
��ʼ(mol): 1 3 0
ת��(mol): x 3x 2x
ƽ��(mol): 1��x 3�� 3x 2x
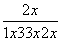 ��100%��42%����x��0.59
��100%��42%����x��0.59
��ƽ��ʱN2���������Ϊ ��100%��14.5%��(4)��ͼʱҪע�ʼʱNH3���ʵ����������࣬����Ϊ��Ӧ�������(��Ӧδ��ƽ��)���ﵽһ���̶Ⱥ�Ӧ�ﵽƽ�����ʱ�¶ȼ������ߣ�ƽ�������ƶ���NH3�����ʵ�����С��(5)�Ƚ���������ʹ��Ҫ���ȵ����ʵõ����ȣ�������ʹ��Ҫ��ȴ�����ʵõ���ȴ���ܳ�������������ϳɰ���ӦΪ�������ʵ�����С�ķ�Ӧ����ѹ���ڷ�Ӧ������У����⣬ѭ�����ÿɷ�������ԭ�ϣ����ԭ�������ʡ�
��100%��14.5%��(4)��ͼʱҪע�ʼʱNH3���ʵ����������࣬����Ϊ��Ӧ�������(��Ӧδ��ƽ��)���ﵽһ���̶Ⱥ�Ӧ�ﵽƽ�����ʱ�¶ȼ������ߣ�ƽ�������ƶ���NH3�����ʵ�����С��(5)�Ƚ���������ʹ��Ҫ���ȵ����ʵõ����ȣ�������ʹ��Ҫ��ȴ�����ʵõ���ȴ���ܳ�������������ϳɰ���ӦΪ�������ʵ�����С�ķ�Ӧ����ѹ���ڷ�Ӧ������У����⣬ѭ�����ÿɷ�������ԭ�ϣ����ԭ�������ʡ�


 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ�ʹ�ò��漰��ѧ�仯����(����)
A����������ˮ������������B��Һ�����������
C��������ʴ���� D����ʯ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ̽����Ȳ����ļӳɷ�Ӧ����ͬѧ��Ʋ����������µ�ʵ�飺��ȡһ������ҵ�õ�ʯ��ˮ��Ӧ�������ɵ�����ͨ����ˮ�У�������Һ��ɫ����֤����Ȳ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У���ɫ�����Һ������������ɫ�Ļ��ǣ��Ʋ����Ƶõ���Ȳ�л����ܺ����������������壬�ɴ�����������ȥ֮��������ˮ��Ӧ������ش��������⣺
(1)д����ͬѧʵ����������Ҫ��Ӧ�Ļ�ѧ����ʽ____________��________________________________________________________��
(2)��ͬѧ��Ƶ�ʵ��(��ܡ����ܡ�)��֤��Ȳ����ˮ�����ӳɷ�Ӧ���������ǡ�
A��ʹ��ˮ��ɫ�ķ�Ӧ��δ���Ǽӳɷ�Ӧ
B��ʹ��ˮ��ɫ�ķ�Ӧ�����Ǽӳɷ�Ӧ
C��ʹ��ˮ��ɫ�����ʣ�δ������Ȳ
D��ʹ��ˮ��ɫ�����ʣ�������Ȳ
(3)��ͬѧ�Ʋ����Ƶõ���Ȳ�к��е����������ǣ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ____________________________________������֤�����б���ȫ����ȥ��
(4)����ѡ����ͼ���е��ĸ�װ��(���ظ�ʹ��)������ͬѧ��ʵ�鷽���������ǵı�����뷽����������д��װ�������ŵĻ�ѧҩƷ��
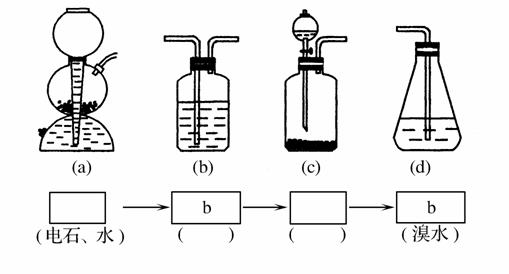
(5)Ϊ��֤��һ��Ӧ�Ǽӳɷ�Ӧ������ȡ����Ӧ����ͬѧ�������pH��ֽ�����Է�Ӧ�����Һ�����ԣ�������_____________________
______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g) 2NO2(g)�����¶����ߣ�����������ɫ���
2NO2(g)�����¶����ߣ�����������ɫ���
�ش��������⣺
(1)��Ӧ�Ħ�H________0(����ڡ���С�ڡ�)��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ________��
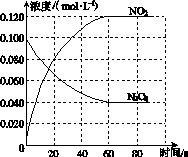
(2)100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣
��T________100 ��(����ڡ���С�ڡ�)���ж�������____________________________��
����ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��_______________________________________
________________________________________________________________________��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����ж�������__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú̿ȼ�չ����л��ͷų�������SO2�������ƻ���̬����������һ����������������Ԫ����CaSO4����ʽ�̶����Ӷ�����SO2���ŷš�����ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ����������Ч�ʡ���ط�Ӧ���Ȼ�ѧ����ʽ���£�
CaSO4(s)��CO(g) CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaSO4(s)��4CO(g) CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
��ش��������⣺
(1)��Ӧ���ܹ��Է����еķ�Ӧ������________��
(2)�����������ķ�Ӧ����ʾƽ�ⳣ��Kpʱ���������(B)��ƽ��ѹǿp(B)������������ʵ�����Ũ��c(B)����Ӧ���Kp��________(�ñ���ʽ��ʾ)��
(3)����ij�¶��£���Ӧ�������(v1 )���ڷ�Ӧ�������(v2 )�������з�Ӧ���������仯ʾ��ͼ��ȷ����________��
(4)ͨ����ⷴӦ��ϵ������Ũ�ȵı仯���жϷ�Ӧ��͢��Ƿ�ͬʱ������������____________________________________________________________________��
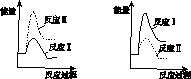
�������������� ��A������������B
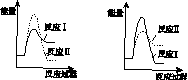
�������������� ��C������������D
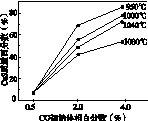
(5)ͼ(a)Ϊʵ���ò�ͬ�¶��·�Ӧ��ϵ��CO��ʼ����ٷ�����ƽ��ʱ���������CaS�����ٷ����Ĺ�ϵ���ߡ��÷�Ӧ��ϵ��SO2�������Ĵ�ʩ��________��
A����÷�Ӧ��ϵ��Ͷ��ʯ��ʯ
B���ں��ʵ��¶�������ƽϵ͵ķ�Ӧ�¶�
C�����CO�ij�ʼ����ٷ���
D����߷�Ӧ��ϵ���¶�
(6)���º��������£����跴Ӧ��͢�ͬʱ��������v1>v2������ͼ(b)������Ӧ��ϵ��c(SO2)��ʱ��t�仯��������ͼ��
 ��
��
����������(a)������������������������(b)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H2O2��һ����ɫ������ԭ�Լ����ڻ�ѧ�о���Ӧ�ù㷺��
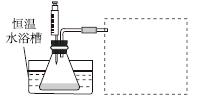
(1)ijС������ͬŨ��Fe3���Ĵ��£�̽��H2O2Ũ�ȶ�H2O2�ֽⷴӦ���ʵ�Ӱ�졣��ѡ�Լ���������30%H2O2��Һ��0.1 mol��L��1Fe2(SO4)3��Һ������ˮ����ƿ��˫������ˮ�ۡ����ܡ��������ܡ���Ͳ�����������ˮԡ�ۡ�ע������
��д����ʵ��H2O2�ֽⷴӦ����ʽ����������ת�Ƶķ������Ŀ��______________________________��
�����ʵ�鷽�����ڲ�ͬH2O2Ũ���£��ⶨ________(Ҫ������õ�������ֱ�����ַ�Ӧ���ʴ�С)��
�����ʵ��װ�ã����ͼ�е�װ��ʾ��ͼ��
�ܲ����±���ʽ���ⶨʵ�������������ʵ�鷽��(�г���ѡ�Լ���������¼�Ĵ��������������ⶨ�����ݣ���������ĸ��ʾ)��
| ���������� ʵ����� ���� | V[0.1 mol��L��1 Fe2(SO4)3]/mL | ���� | |
| 1 | a | ���� | |
| 2 | a | ���� |
(2)����ͼ(a)��(b)�е���Ϣ����ͼ(c)װ��(��ͨ��A��Bƿ���ѳ���NO2����)����ʵ�顣�ɹ۲쵽Bƿ��������ɫ��Aƿ�е�__________(����dz��)����ԭ����____________________________��
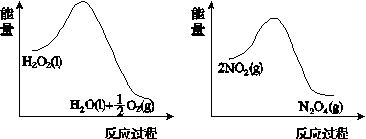
����������(a)������������������������(b)
(c)
ͼ21
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�

(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2.0 L�����ܱ������г���1.0 mol PCl5�����¶�ΪTʱ������ӦPCl5(g) 
 PCl3(g)��Cl2(g)����H����124 kJ��mol��1����Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)��Cl2(g)����H����124 kJ��mol��1����Ӧ�����вⶨ�IJ������ݼ��±���
| ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
�ش��������⣺
(1)��Ӧ��ǰ50 s��ƽ������v(PCl5)��____________��
(2)�¶�ΪTʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ����________��
(3)Ҫ���������Ӧ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��______________��
(4)���¶�ΪTʱ������ʼʱ�������г���0.5 mol PCl5��a mol Cl2ƽ��ʱPCl5��ת������Ϊ20%����a��________��
(5)����ˮ�У����Ȼ�����ȫˮ�⣬��������(H3PO4)���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
����0.01 mol PCl5Ͷ��1 L��ˮ�У�����μ���AgNO3��Һ���Ȳ����ij�����________[��֪Ksp(Ag3PO4)��1.4��10��16��Ksp(AgCl)��1.8��10��10]��
(6)һ�������£���������������Һ����һ�ֵ������������壬���ɸ����������ĵİ������ʵ���֮��Ϊ20��3����Ӧ�Ļ�ѧ����ʽΪ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
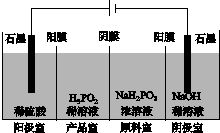
��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com