
����Ŀ��̼���仯�����ڻ������������Ź㷺��Ӧ�á�
I.Ϊ���������CO2�ĺ�����������⣬ij��ѧ��������¹��룺
�ѹ����ų��ĸ���CO2�ķ�������������̼�����Һ���գ�Ȼ���ٰ�CO2����Һ����ȡ�������ںϳ����о���ѧ��Ӧʹ�����е�CO2ת��Ϊȼ�ϼ״���
���ּ�����������:
�źϳ����з�Ӧ�Ļ�ѧ����ʽΪ____________����H<0���÷�ӦΪ���淴Ӧ����ƽ���ƶ�ԭ���������������������ԭ������ƽ��ת���ʡ���ʵ�������в���300�����¶ȣ��������¶ȶԷ�Ӧ���ʵ�Ӱ���⣬����Ҫ������___________________________________________________________________��
(2)�Ӻϳ���������״���ԭ��������_______������ԭ���Ƚ����(����ĸ��
A.���� B.��Һ C.���� D.�ᾧ
(3)�罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��д��CO2(g)��H2(g)��Ӧ��CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ:
��֪��CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H1=-890.3kJ/mol
2H2(g)+O2(g)=2H2O(l) ��H2=-571.6kJ/mol
_______________________________________________________________________��
II.����ȼ�ջ�ų��������ȣ�����Ϊ��ԴӦ������������������
��֪����2CH4(g)+3O2(g)=2CO(g)+4H2O(l)�� ��H1=-1214.6kJ/mol
��CO2(g)=CO(g)+1/2O2(g)�� ��H2=+283.0kJ/mol
���ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ______________________________��
III.ij��ȤС��ģ�ҵ�ϳɼ״��ķ�Ӧ:CO(g)+2H2(g)![]() CH3OH(g)�����ݻ��̶�Ϊ2L���ܱ������г���1mol CO 2mol H2��������ʵĴ���������������Բ��ƣ���ʼ��Ӧ����������ڵ�ѹǿ��ʱ��仯����:
CH3OH(g)�����ݻ��̶�Ϊ2L���ܱ������г���1mol CO 2mol H2��������ʵĴ���������������Բ��ƣ���ʼ��Ӧ����������ڵ�ѹǿ��ʱ��仯����:
ʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
ѹǿ/Mpa | 12.6 | 10.8 | 9.5 | 8.7 | 8.4 | 8.4 |
(1)�ӷ�Ӧ��ʼ��20minʱ����CO��ʾ��Ӧ����Ϊ_____________________��
(2)����������˵����Ӧ�ﵽƽ�����_______________________
A.װ����������ɫ���ٸı� B.�����������ƽ��Ħ���������ֲ���
C.�����������ѹǿ���ֲ��� D.�����������ܶȱ��ֲ���
(3)���¶���ƽ�ⳣ��K=____�����ﵽƽ����������CH3OH(g)����ʱƽ�ⳣ��Kֵ��____ (����������������С������������)
(4)�÷�Ӧ�ﵽƽ������������г���1mol CO 2mol H2����ʱCO��ת���ʽ�__________(����������������С������������)
���𰸡� CO2+3H2![]() CH3OH+H2O �����Ĵ����ԡ���Ӧ���� C CO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=-252.9kJ/mol CH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=-890.3kJ/mol 0.0125mol��L-1��min-1 BC 4 ���� ����
CH3OH+H2O �����Ĵ����ԡ���Ӧ���� C CO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=-252.9kJ/mol CH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=-890.3kJ/mol 0.0125mol��L-1��min-1 BC 4 ���� ����
��������������������⿼�鷴Ӧ�ȵļ��㣬�Ȼ�ѧ����ʽ����д����ѧ��Ӧ���ʵļ��㣬��ѧƽ����жϣ���������Ի�ѧ��Ӧ���ʺͻ�ѧƽ���Ӱ���Լ�ʵ��Ӧ�á�
I��1������CO2�ķ�����������̼�����Һ���շ�����ӦCO2+K2CO3+H2O=2KHCO3���ڷֽ���з�����Ӧ2KHCO3![]() K2CO3+H2O+CO2�����ϳ�������CO2��H2��2
K2CO3+H2O+CO2�����ϳ�������CO2��H2��2![]() 105Pa��300�桢��п��ý�����·�Ӧ����CH3OH����Ӧ�Ļ�ѧ����ʽΪCO2+3H2
105Pa��300�桢��п��ý�����·�Ӧ����CH3OH����Ӧ�Ļ�ѧ����ʽΪCO2+3H2![]() CH3OH+H2O���÷�Ӧ�Ħ�H
CH3OH+H2O���÷�Ӧ�Ħ�H![]() 0������������ƽ�������ƶ������ԭ�ϵ�ת���ʣ�����300����˿����¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�������¶ȶԴ��������ԡ���Ӧ���ʵ�Ӱ�졣
0������������ƽ�������ƶ������ԭ�ϵ�ת���ʣ�����300����˿����¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�������¶ȶԴ��������ԡ���Ӧ���ʵ�Ӱ�졣
��2��������CH3OH��H2O������ܣ��Ӻϳ��������CH3OH�� ��������������IJ���ԭ����
��3����CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H1=-890.3kJ/mol��2H2(g)+O2(g)=2H2O(l) ��H2=-571.6kJ/mol���α�Ϊ�١��ڣ����ø�˹���ɣ���![]() 2-����CO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=��-571.6kJ/mol��
2-����CO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=��-571.6kJ/mol��![]() 2-��-890.3kJ/mol��=-252.9kJ/mol����Ӧ���Ȼ�ѧ����ʽΪCO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=-252.9kJ/mol��
2-��-890.3kJ/mol��=-252.9kJ/mol����Ӧ���Ȼ�ѧ����ʽΪCO2(g)+4H2(g) = CH4(g)+2H2O(l) ��H=-252.9kJ/mol��
II���ø�˹���ɣ���![]() -������CH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=��-1214.6kJ/mol��
-������CH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=��-1214.6kJ/mol��![]() -��+283.0kJ/mol��=-890.3kJ/mol����CH4ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=-890.3kJ/mol��
-��+283.0kJ/mol��=-890.3kJ/mol����CH4ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g) = CO2(g)+2H2O(l) ��H=-890.3kJ/mol��
III��1����������ʽ�������跴Ӧ��ʼ��20min��ת����CO���ʵ���Ϊxmol����
CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
n����ʼ����mol�� 1 2 0
n��ת������mol�� x 2x x
n��20minĩ����mol��1-x 2-2x x
���ݺ��º���ʱ����ѹǿ֮�ȵ������ʵ���֮�ȣ���ʽ![]() =
=![]() �����x=0.5����ӷ�Ӧ��ʼ��20min�ڦ���CO��=0.5mol
�����x=0.5����ӷ�Ӧ��ʼ��20min�ڦ���CO��=0.5mol![]() 2L
2L![]() 20min=0.0125mol��L-1��min-1��
20min=0.0125mol��L-1��min-1��
��2��A�CO��H2��CH3OH��g��������ɫ���壬��ɫ���ٱ仯����˵����Ӧ�Ѵ�ƽ�⣬����B������������ʶ�����̬�������������ʼ�ղ��䣬����ӦΪ�����������С�ķ�Ӧ������ƽ��Ĺ��������������ʵ�����С�������ƽ��Ħ���������������ƽ��Ħ���������������Ӧ�ﵽƽ��״̬����ȷ��C����º���ʱ����ѹǿ֮�ȵ����������ʵ���֮�ȣ�����ӦΪ�����������С�ķ�Ӧ������ƽ��Ĺ��������������ʵ�����С���������ѹǿ��С�������ѹǿ��������ﵽƽ��״̬����ȷ��D������������ʶ�����̬�������������ʼ�ղ��䣬������������䣬��������ܶ�ʼ�ղ��䣬��������ܶȲ��䲻����Ϊƽ��ı�־������ѡBC��
��3��20min��25minʱѹǿ����˵���Ѿ��ﵽƽ��״̬��������������֪ƽ��ʱCO��H2��CH3OH��g����Ũ������Ϊ0.25mol/L��0.5mol/L��0.25mol/L������¶��µĻ�ѧƽ�ⳣ��K=![]() =4����ѧƽ�ⳣ��Kֻ���¶��й������ﵽƽ����������CH3OH(g)��Kֵ���䡣
=4����ѧƽ�ⳣ��Kֻ���¶��й������ﵽƽ����������CH3OH(g)��Kֵ���䡣
��4���÷�Ӧ�ﵽƽ������������г���1mol CO��2mol H2���൱������ѹǿ��ƽ�������ƶ���CO��ת��������


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һһ�������Ե���
A.ʹ������Һ�ʻ�ɫ����ҺB.c(H+)= c(OH-)=10-6mol/L ��Һ
C.pH=7 ����ҺD.�����ǡ����ȫ��Ӧ�������ε���Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�������ʾ��ͼһ�µ���
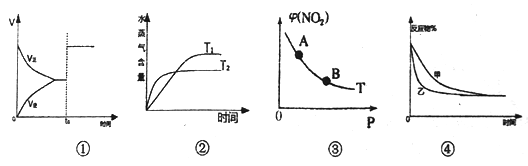
A. ͼ�ٱ�ʾaA(g)+bB(g)![]() cC(g)��Ӧ������ʱ��仯��ʾ��ͼ����toʱ�̸ı������һ��Ϊ�����������
cC(g)��Ӧ������ʱ��仯��ʾ��ͼ����toʱ�̸ı������һ��Ϊ�����������
B. ͼ�ڱ�ʾ��ӦH2(g)+CO2(g)![]() CO(g)+H2O(g)���ڲ�ͬ�¶��£�ˮ����������ʱ��ı仯����÷�Ӧ��H>0
CO(g)+H2O(g)���ڲ�ͬ�¶��£�ˮ����������ʱ��ı仯����÷�Ӧ��H>0
C. ͼ�۱�ʾ2NO2(g)![]() N2O4(g)�ﵽƽ��ʱ��NO2���������ѹǿ�仯ʾ��ͼ�����У�B�����ɫ��A�����ɫ��
N2O4(g)�ﵽƽ��ʱ��NO2���������ѹǿ�仯ʾ��ͼ�����У�B�����ɫ��A�����ɫ��
D. ͼ�ܱ�ʾѹǿ��2A(g)+3B(g)![]() 3C(g)+2D(s)��Ӱ�죬���ҵ�ѹǿ�ȼ�ѹǿ��
3C(g)+2D(s)��Ӱ�죬���ҵ�ѹǿ�ȼ�ѹǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������У����ܰ���ͼ����������ʾһ����ɣ���ϵ�ת�����ǣ� ��
ѡ�� | X | Y | Z |
A | Fe | FeCl3 | FeCl2 |
B | Cl2 | NaClO | NaCl |
C | Si | SiO2 | H2SiO3 |
D | HNO3 | NO | NO2 |

A.A
B.B
C.C
D.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��������Ƽ���ϵ���У����н�����ȷ����
A. �����������������µ���Ҫԭ���Ƿŵ�ʱN2��O2�ϳ���NO
B. ά����D�ܴٽ�����ԸƵ�����
C. ��̼����������й¶��ԭ��ʱ�����˻�ѧ�仯
D. ���������Ľ������֬���������Ǹ߷�������С���ӵĹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2H4��һ�ָ�Ч���Ļ��ȼ�ϡ�8g N2H4(g)��ȫȼ�����ɵ�������̬ˮʱ���ų�133.5 kJ�������������Ȼ�ѧ����ʽ����ȷ����(����)
A. ![]() N2H4(g)��O2(g)=== N2(g)��H2O(g)�� ��H��267 kJ��mol��1
N2H4(g)��O2(g)=== N2(g)��H2O(g)�� ��H��267 kJ��mol��1
B. N2H4(g)��O2(g)===N2(g)��2H2O(g)�� ��H����534 kJ��mol��1
C. N2H4(g)��O2(g)===N2(g)��2H2O(g)�� ��H��534 kJ��mol��1
D. N2H4(g)��O2(g)===N2(g)��2H2O(l)�� ��H����133.5 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�϶������Ե����� ��
A. �ͽ�������Ӧ������������Һ B. �ӷ�̪����ɫ����Һ
C. pH<7����Һ D. c��H+����c��OH-������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2 L���ܱ���������3�����ʽ��з�Ӧ��X��Y��Z�����ʵ�����ʱ��仯��������ͼ��ʾ����Ӧ��t1minʱ�ﵽ��ѧƽ��״̬��
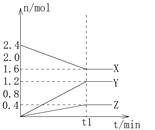
(1) 0��t1min�ڣ�X��Ũ�ȱ仯��Ϊ___________Y��Ũ�ȱ仯��______________
(2) 0��t1min�ڣ�Y��ƽ����Ӧ����Ϊ_________________________________��
(3) X��Y��Z���ߵ�����֮��Ϊ_____________________��
(4) �÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
(5) ���й��ڸ÷�Ӧ��˵����ȷ����__________________________________��
A��t1minʱ���÷�Ӧ��ֹͣ
B��t1min֮ǰ��X���������ʴ���������������
C��t1minʱ������Ӧ���ʵ����淴Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У������÷�Һ©���������
A.�������ˮB.�ױ���ˮC.ֲ���ͺ�ˮD.�Ҵ���ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com