
 �ⶨ���������ڱ�״���µ������V1L��
�ⶨ���������ڱ�״���µ������V1L��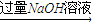 ��ַ�Ӧ��ⶨʣ������������W1g��
��ַ�Ӧ��ⶨʣ������������W1g�� ��Һ
��Һ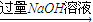 ���ˣ��ⶨ������������W2g��
���ˣ��ⶨ������������W2g�� �ⶨ���������ڱ�״���µ������V2L����
�ⶨ���������ڱ�״���µ������V2L����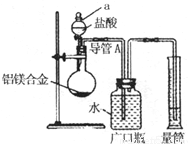
 mol��
mol��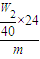 ×100%=
×100%=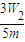 ×100%
×100% ×100%��
×100%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����
��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����| �������� |
| ����NaOH��Һ |
| �������� |
| ����NaOH��Һ |
| 3W2 |
| 5m |
| 3W2 |
| 5m |
| () |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| NaOH��Һ |
| ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
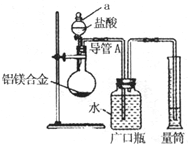 ��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����
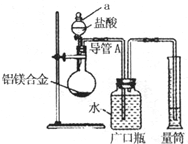
��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽���� �ⶨ���������ڱ�״���µ������V1L��
�ⶨ���������ڱ�״���µ������V1L�� ��ַ�Ӧ��ⶨʣ������������W1g��
��ַ�Ӧ��ⶨʣ������������W1g�� ��Һ
��Һ ���ˣ��ⶨ������������W2g��
���ˣ��ⶨ������������W2g�� �ⶨ���������ڱ�״���µ������V2L����
�ⶨ���������ڱ�״���µ������V2L�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
 �ⶨ����������������״����
�ⶨ����������������״����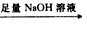 �ⶨ����������������״����
�ⶨ����������������״����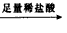 ��Һ
��Һ ���ˣ��ⶨ����������
���ˣ��ⶨ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�밴Ҫ��ش���������:
��1��.ij����ѧϰС�����۱�������˵��������˵����ȷ���� _____________
A����������к��е���ԭ�Ӹ����������ΪһԪ�ᡢ��Ԫ���
B ��Na2O2Ͷ��FeCl2��Һ��, �ɹ۲쵽�����������ɺ��ɫ�����������ݲ���
C ����Ԫ�ص�ԭ��ֻ�л�ԭ�ԣ�����ֻ��������
D����ֺ�Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
E NH3��CO2��ˮ��Һ���ܵ��磬����NH3��CO2���ǵ����
��2����5mol/L��Mg��NO3��2��Һa mLϡ����b mL��ϡ�ͺ���Һ��NO3�������ʵ���Ũ��Ϊ_____________
��3����K2Cr2O7+14HCl==2KCl+2CrCl3+3Cl2��+7H2O��Ӧ�У�
��������_____ _���ѧʽ�� ����14.6gHCl������ʱ������ת��Ϊ mol��
��4��ѧУ�о���ѧϰС���ͬѧ��Ϊ�ⶨij��þ3%��5%����þ�Ͻ�(��������Ԫ��)��þ�������������������ʵ�鷽������̽������д���пհף�
ʵ�鷽������þ�Ͻ� ![]()
![]() �ⶨʣ�����������
�ⶨʣ�����������
�� ʵ���з�����Ӧ�Ļ�ѧ����ʽ��__________________��
�� ʵ�鲽�裺��ȡ5.4 g��þ�Ͻ��ĩ��Ʒ��Ͷ��V mL 2.0 mol/L NaOH ��Һ�г�ַ�Ӧ����������NaOH��Һ�����V��______________��
���ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������________( �� ���� ƫ�� �� ƫ�� )��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com