
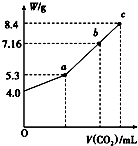
 Ķł100mLµÄNaOHČÜŅŗÖŠĶØČėCO2³ä·Ö·“Ó¦ŗó£¬ŌŚ¼õŃ¹ŗĶ½ĻµĶĪĀ¶ČĻĀ£¬Š”ŠÄµŲ½«ČÜŅŗÕōøÉ£¬µĆµ½°×É«¹ĢĢåM£¬ĶØČėCO2µÄĢå»ż£Ø±ź×¼×“æö£©ÓėMµÄÖŹĮæWµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
Ķł100mLµÄNaOHČÜŅŗÖŠĶØČėCO2³ä·Ö·“Ó¦ŗó£¬ŌŚ¼õŃ¹ŗĶ½ĻµĶĪĀ¶ČĻĀ£¬Š”ŠÄµŲ½«ČÜŅŗÕōøÉ£¬µĆµ½°×É«¹ĢĢåM£¬ĶØČėCO2µÄĢå»ż£Ø±ź×¼×“æö£©ÓėMµÄÖŹĮæWµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö ÓÉĶ¼ÖŖNaOHÖŹĮæĪŖ4 g£¬ĪļÖŹµÄĮæĪŖ0.1 mol£¬øł¾Żc=$\frac{n}{V}$¼ĘĖć£»ĶźČ«×Ŗ»ÆĪŖNa2CO3Ź±£¬Na2CO3ÖŹĮæĪŖ0.1mol”Į$\frac{1}{2}$”Į106g/mol=5.3 g£¬ĶźČ«×Ŗ»ÆĪŖNaHCO3Ź±£¬NaHCO3ÖŹĮæĪŖ0.1mol”Į84g/mol=8.4 g£¬¹ŹAµć°×É«¹ĢĢåMĪŖNa2CO3£¬Cµć°×É«¹ĢĢåMĪŖNaHCO3£¬øł¾ŻĢ¼Ō×ÓŹŲŗćæɵĆn£ØCO2£©£¬øł¾ŻV=nVm¼ĘĖ涞Ńõ»ÆĢ¼Ģå»ż£»
Ķ¼BµćŹ±MµÄÖŹĮæĪŖ7.16 g£¬5.3£¼7.16£¼8.4£¬ÖŖMÓÉNa2CO3ŗĶNaHCO3×é³É£¬ÉčŌŚBµćŹ±Na2CO3ĪļÖŹµÄĮæĪŖx£¬NaHCO3ĪļÖŹµÄĮæĪŖy£¬øł¾ŻÄĘĄė×ÓŹŲŗć”¢¶žÕßÖŹĮæÖ®ŗĶĮŠ·½³Ģ¼ĘĖćx”¢yµÄÖµ£¬øł¾ŻV=nVm¼ĘĖ涞Ńõ»ÆĢ¼Ģå»ż£»øł¾ŻNaHCO3+NaOH=Na2CO3+H2O¼ĘĖć£®
½ā“š ½ā£ŗÓÉĶ¼ÖŖNaOHÖŹĮæĪŖ4 g£¬ĪļÖŹµÄĮæĪŖ0.1 mol£¬ĶźČ«×Ŗ»ÆĪŖNa2CO3Ź±£¬Na2CO3ÖŹĮæĪŖ0.1mol”Į$\frac{1}{2}$”Į106g/mol=5.3 g£¬ĶźČ«×Ŗ»ÆĪŖNaHCO3Ź±£¬NaHCO3ÖŹĮæĪŖ0.1mol”Į84g/mol=8.4 g£¬¹ŹAµć°×É«¹ĢĢåMĪŖNa2CO3£¬Cµć°×É«¹ĢĢåMĪŖNaHCO3£¬BµćŹ±£¬°×É«¹ĢĢåMµÄ»ÆѧŹ½ĪŖNa2CO3”¢NaHCO3£»
£Ø1£©ÓÉĶ¼ÖŖ£¬¶žŃõ»ÆĢ¼Ģå»żĪŖ0Ź±£¬¹ĢĢåµÄÖŹĮæĪŖ4g£¬¼“NaOHÖŹĮæĪŖ4 g£¬ĪļÖŹµÄĮæĪŖ0.1 mol£¬Ōņc=$\frac{n}{V}$=$\frac{0.1mol}{0.1L}$=1mol/L£»
¹Ź“š°øĪŖ£ŗ1mol/L£»
£Ø2£©ÓÉÉĻŹö·ÖĪöæÉÖŖ£¬Aµć°×É«¹ĢĢåMĪŖNa2CO3£¬ŠčCO2Ģå»żĪŖ0.1 mol”Į$\frac{1}{2}$”Į22.4L•mol-1=1.12L=1120 mL£¬
¹Ź“š°øĪŖ£ŗNa2CO3£»1120£»
£Ø3£©Ķ¼BµćŹ±MµÄÖŹĮæĪŖ7.16 g£¬5.3£¼7.16£¼8.4£¬ÖŖMÓÉNa2CO3ŗĶNaHCO3×é³É£¬ÉčŌŚBµćŹ±Na2CO3ĪļÖŹµÄĮæĪŖx£¬NaHCO3ĪļÖŹµÄĮæĪŖy£¬
Ōņ$\left\{\begin{array}{l}{106x+84y=7.16}\\{2x+y=0.1}\end{array}\right.$£¬½āµĆx=0.02£¬y=0.06£¬
¹ŹV£ØCO2£©=£Ø0.02 mol+0.06 mol£©”Į22.4L•mol-1=1.792L£»
¹Ź“š°øĪŖ£ŗNa2CO3”¢NaHCO3£»1.792£»
£Ø4£©ČōĻņÉś³ÉµÄ7.16gŃĪµÄČÜŅŗÖŠ¼ÓČėŅ»¶ØĮæµÄNaOH£¬³ä·Ö·“Ó¦ŗ󣬼õŃ¹µĶĪĀÕō·¢µĆµ½“æ¾»µÄĢ¼ĖįÄĘ¹ĢĢå£ØĪŽ½į¾§Ė®£©8.4g£¬Ōņ·¢Éś·“Ó¦ĪŖNaHCO3+NaOH=Na2CO3+H2O£¬ŅŃÖŖNaHCO3ĪļÖŹµÄĮæĪŖ0.06mol£¬Ōņ¼ÓČėµÄĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæŹĒ0.06mol£»
¹Ź“š°øĪŖ£ŗ0.06mol£®
µćĘĄ ±¾Ģāæ¼²éĮĖ»ģŗĻĪļ·“Ó¦µÄ¼ĘĖć£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·³£¼ūŌŖĖŲ¼°Ęä»ÆŗĻĪļŠŌÖŹĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕżČ··ÖĪöĶ¼ĻóÖŠĒśĻßø÷µć¶ŌÓ¦ČÜÖŹ×é³É£¬ŹŌĢāÓŠĄūÓŚÅąŃųѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦¼°Įé»īÓ¦ÓĆÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | O2”¢SO2 | B£® | NH3”¢N2 | C£® | NO”¢O2 | D£® | NH3”¢HCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ī½šŹōµ„ÖŹÓėĖ®·“Ó¦¶¼ÄÜÉś³É¼īŗĶH2 | |
| B£® | ¼ī½šŹōµ„ÖŹ¶¼ŹĒÖŹČķ”¢µēŗĶČȵÄĮ¼µ¼Ģ壬ŃęÉ«·“Ó¦¶¼³ŹĻÖ»ĘÉ« | |
| C£® | ¼ī½šŹōµÄĆܶȶ¼Š”ÓŚ1g/cm3£¬Ņņ“Ė¼ī½šŹōµ„ÖŹ¶¼æÉŅŌ±£“ęŌŚĆŗÓĶÖŠ | |
| D£® | ¼ī½šŹōµ„ÖŹŌŚæÕĘųÖŠČ¼ÉÕ¶¼Éś³É¹żŃõ»ÆĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ¼ÓČČ·½Ź½ | ²śĪļŌŖĖŲ×é³É | ø÷ŌŖĖŲµÄÖŹĮæ·ÖŹż/% | |
| Fe | O | ||
| ¾Ę¾«µĘ | FeŗĶO | 74.50 | 25.50 |
| “ųĶųÕÖ¾Ę¾«µĘ | FeŗĶO | 76.48 | 23.52 |
| ¾Ę¾«ÅēµĘ | Fe | 100.00 | 0.00 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ģś¼°Ęä»ÆŗĻĪļŌŚČÕ³£Éś»ī”¢Éś²śÖŠÓ¦ÓĆ¹ć·ŗ£¬ŃŠ¾æĢś¼°Ęä»ÆŗĻĪļµÄÓ¦ÓĆŅāŅåÖŲ“ó£®»Ų“šĻĀĮŠĪŹĢā£ŗ
Ģś¼°Ęä»ÆŗĻĪļŌŚČÕ³£Éś»ī”¢Éś²śÖŠÓ¦ÓĆ¹ć·ŗ£¬ŃŠ¾æĢś¼°Ęä»ÆŗĻĪļµÄÓ¦ÓĆŅāŅåÖŲ“ó£®»Ų“šĻĀĮŠĪŹĢā£ŗ| ĪĀ¶Č | 250”ę | 600”ę | 1000”ę | 2000”ę |
| Ö÷ŅŖ³É·Ö | Fe2O3 | Fe3O4 | FeO | Fe |
| t/min | 0 | 10 | 20 | 30 | 40 | 50 |
| x£ØCO£© | 0.25 | 0.23 | 0.214 | 0.202 | 0.193 | 0.193 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚŅ½ĮĘÉĻĢ¼ĖįÄĘ”¢Al£ØOH£©3¾łæÉÓĆÓŚÖĪĮĘĪøĖį¹ż¶ą | |
| B£® | ČĖŌģøÕÓńµÄČŪµćŗÜøߣ¬æÉÓĆ×÷øß¼¶ÄĶ»š²ÄĮĻ£¬ĘäÖ÷ŅŖ³É·ÖŹĒSiO2 | |
| C£® | Ė®²£Į§æÉÓĆÓŚÉś²śÕ³ŗĻ¼ĮŗĶ·Ą»š¼Į | |
| D£® | ×ŌĄ“Ė®ÖŠ¼ÓČėÉŁĮæĆ÷·Æ£¬Ė®½āÉś³ÉAl£ØOH£©3½ŗĢåæÉŅŌĘšµ½É±¾śĻū¶¾µÄ×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com