
ijѧ����NaHCO3��KHCO3��ɵĻ�������ʵ�飬�����������(ÿ�μ������������ʵ���Ũ�����)�����з�����ȷ����(����)
| ����/mL | 50 | 50 | 50 |
| m(�����)/g | 9.2 | 15.7 | 27.6 |
| V(CO2)(��״��)/L | 2.24 | 3.36 | 3.36 |
A����������ʵ���Ũ��Ϊ3.0 mol��L��1
B���������NaHC O3����������Ϊ54.3%
O3����������Ϊ54.3%
C��9.2 g�������KHCO3���� �ʵ���Ϊ0.05 mol
�ʵ���Ϊ0.05 mol
D��15.7 g�����ǡ����������ȫ��Ӧ
������ѡAC����m��27.6 gʱ�����������㣬��������������������������H����HCO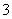 ===CO2����H2O֪��n(HCl)��3.36 L��22.4 L��mol��1��0.15 mol��c(HCl)��0.15 mol��0.05 L�� 3.0 mol��L��1��A����ȷ����m��9.2 gʱ�������������9.2 g������У�NaHCO3��KHCO3�����ʵ����ֱ�Ϊn(NaHCO3)��n(KHCO3)�����У�n(NaHCO3)��n(KHCO3)��0.1 mol,84 g��mol��1 ��n(NaHCO3)��100 g��mol��1��n(KHCO3)��9.2 g��������ʽ��ã�n(NaHCO3)��n(KHCO3)��0.05 mol��C����ȷ��NaHCO3����������С��KHCO3������������B���ȷ������������ʱ������3.36 L�����������������Ϊ9.2 g��1.5��13.8 g��D���ȷ��
===CO2����H2O֪��n(HCl)��3.36 L��22.4 L��mol��1��0.15 mol��c(HCl)��0.15 mol��0.05 L�� 3.0 mol��L��1��A����ȷ����m��9.2 gʱ�������������9.2 g������У�NaHCO3��KHCO3�����ʵ����ֱ�Ϊn(NaHCO3)��n(KHCO3)�����У�n(NaHCO3)��n(KHCO3)��0.1 mol,84 g��mol��1 ��n(NaHCO3)��100 g��mol��1��n(KHCO3)��9.2 g��������ʽ��ã�n(NaHCO3)��n(KHCO3)��0.05 mol��C����ȷ��NaHCO3����������С��KHCO3������������B���ȷ������������ʱ������3.36 L�����������������Ϊ9.2 g��1.5��13.8 g��D���ȷ��


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2mol/L��NaOH��Һ�ζ�pH=5��HCN��Һ100mL�����ԣ���ʱ��Һ�и�����Ũ�ȹ�ϵ��ȷ����
A. c(Na��)>c(CN��)>c(OH��)>c(H��) B��c(CN��)>c(Na��)>c(H��)> c(OH��)
C. c(Na��)+c(CN��)=2mol��L��1 D��c(Na��)+c(H+)= c(CN��)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100 mL�廯������Һ�л���ͨ��2.24 L(��״����)Cl2,��Ӧ��ȫ����Һ����1/3�������ӱ������ɵ����塣��ԭ�廯������Һ�����ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����е�3He�Ǻ˾۱䷢��������ȫ���������ʣ������Ϻ�Ԫ����Ҫ��4He��ʽ���ڣ�3He����15 t���ң��������ϵ�3He���������֮�࣬�ɹ�ȫ���翪��500�ꡣ����˵����ȷ����(����)
��3He��4He�Ļ�ѧ���ʻ�����ͬ����3He��4He������ͬ������������3He�˾۱��ǻ�ѧ�仯����3HeҺ���������仯����3He��4He�γɵĵ����о����й��ۼ�����3He��4He�ֱ���ɵ����嵥�ʣ�����ͬ�������ܶ�֮��Ϊ3��4
A���٢ڢ� B���٢ܢ�
C���ڢۢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶Ⱥ�ѹǿ�£�30 Lij����̬�������к���6.02��1023�����ӣ���Щ ������1.204��1024��ԭ����ɣ������й�˵���в���ȷ����(����)
������1.204��1024��ԭ����ɣ������й�˵���в���ȷ����(����)
A�����¶Ⱥ�ѹǿ�����DZ�״��
B����״���¸ô�������Ϊ��̬�������Լ��22.4 L
C����������ÿ�����Ӻ���2��ԭ��
D����O2�ڸ�������Ϊ��̬����1 mol O2�ڸ������µ����Ϊ22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ƣ���������Ӻ���(xNa2SO4��yH2O2��zH2O)����ɿ�ͨ������ʵ��ⶨ����ȷ��ȡ1.770 0 g��Ʒ�����Ƴ�100.00 mL��ҺA����ȷ��ȡ25.00 mL��ҺA�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����0.5825 g����ȷ��ȡ25.00 mL��ҺA��������ϡ�����ữ����0.020 00 mol��L��1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ25.00 mL��H2O2��KMnO4��Ӧ�����ӷ���ʽ���£�2MnO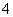 ��5H2O2��6H��===2Mn2����8H2O��5O2��
��5H2O2��6H��===2Mn2����8H2O��5O2��
(1)��֪������BaSO4��Ksp��1.1��10��10����ʹ��Һ��c(SO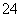 )��1.0��10��6 mol��L��1��Ӧ��
)��1.0��10��6 mol��L��1��Ӧ�� ����Һ��c(Ba2��)��________mol��L��1��
����Һ��c(Ba2��)��________mol��L��1��
(2)�����ζ�������ϡ�����ữ��MnO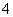 ����ԭΪMnO2�������ӷ���ʽΪ______________��
����ԭΪMnO2�������ӷ���ʽΪ______________��
(3)ͨ������ȷ����Ʒ�����(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж��л�������ķ�������ȷ����
(����)
A����ϩCH2===CH2�����������鶼����֬����
B������������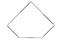 ��������ͬ���ڷ�����
��������ͬ���ڷ�����
C����ϩCH2===CH2����ȲCHCHͬ����ϩ��
D��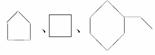 ͬ���ڻ�����
ͬ���ڻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������̼ԭ��Ϊ���ĵ���������ṹ�����������ε�ƽ��ṹ����������
(���� )
A��CHCl3ֻ��һ�ֽṹ
B��CH2Cl2ֻ��һ�ֽṹ
C��CH4���м��Լ�
D��CH4���ĸ��ۼ��ļ����ͼ��ܶ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ֺ��ؽ��У�����ˮ��ȫռ�м�Ϊ��Ҫ�ĵ�λ�� ij�о�С����ȡ��������Ⱦ��ˮԴ���з�����������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��E���ֳ��������ﶼ�����±��е������γɵģ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�У����ְ�ɫ�����������μӳ����ܽ⡣
�۽�����ɫ��Ӧʵ�飬ֻ��B��C���м����ӡ�
���ڸ���Һ�м���Ba(NO3)2��Һ���ټ������ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ��������д���пհף�
(1)д��B��C��D�Ļ�ѧʽ��B________��C________��D________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ϊ________��
(3)д��ʵ��ڷ�����Ӧ�����ӷ���ʽ________________________________________
________________________________________________________________________��
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��_______________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com