
A��B��C��D��Eԭ���������������Ҿ�Ϊ������Ԫ�ء���֪��A��������������������Ӳ�����B�������������Ǵ�����������������C��Dͬ�������ڣ�D��Eͬ�������ڣ�A��C��D����Ԫ�ص�������֮����EԪ�ص���������ȣ�AԪ����B��C��D��E����Ԫ�ؼȲ�ͬ����Ҳ��ͬ���塣��ش�
��1��A��Ԫ�ط����� ������E��ԭ�ӽṹʾ��ͼ ��
��2��д����A��C��D��4��2��3��ɵ����ӻ�����Ļ�ѧʽ �������ӻ������г��������Ӽ������ ����д����A��B��C��D��4��1��?2��1?��ɵ�һ�ֻ��ʵ����ƣ������� ��
��3��B��D��ɻ�����BD2�ĵ���ʽΪ ��A��C��ɵĹ��ۻ�����C2A4��������ƽ�����д���仯ѧʽ ��
��1��H ![]()
��2��NH4NO3����������Ҳ��ȷ�� ���� ?����
��3��![]()
�ڶ�������������������������Ӳ�����Ԫ����H��Be��Al�������������Ǵ�����������������Ԫ��ΪC����BΪ̼Ԫ�أ�����AӦΪHԪ�ػ���BeԪ�أ��ɼ���ΪHԪ�أ�����DԪ�ص�������Ϊm����CԪ�ص�������Ϊm��1��EԪ�ص�������Ϊm��8��������m��8=m����m��1����1,���m=8����CΪNԪ�أ�DΪOԪ�أ�EΪSԪ�ء�


 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
 Al��OH��3+3H+
Al��OH��3+3H+ Al��OH��3+3H+
Al��OH��3+3H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
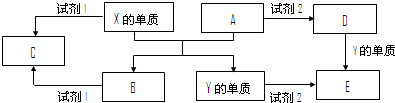
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
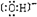
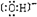
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��E���ֶ�����Ԫ�أ�ԭ������E��D��A��B��C����A��B��D��Eͬ���ڣ�A��Cͬ���壬A��ԭ�ӽṹʾ��ͼ��ͼ��B��������������K����Ӷ�1��DԪ�ص���������������������2����E�ĵ����ǻ���ɫ���壻�ݴ���գ�
A��B��C��D��E���ֶ�����Ԫ�أ�ԭ������E��D��A��B��C����A��B��D��Eͬ���ڣ�A��Cͬ���壬A��ԭ�ӽṹʾ��ͼ��ͼ��B��������������K����Ӷ�1��DԪ�ص���������������������2����E�ĵ����ǻ���ɫ���壻�ݴ���գ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
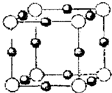 ��2012?����ģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������Aԭ�Ӻ���������δ�ɶԵ��ӣ�A��B���γ����ӻ�����B3A2��CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�Dԭ������M���������ԳɶԵ��ӣ�Eԭ�Ӻ��������ֻ��1�����ӣ����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��2012?����ģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������Aԭ�Ӻ���������δ�ɶԵ��ӣ�A��B���γ����ӻ�����B3A2��CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�Dԭ������M���������ԳɶԵ��ӣ�Eԭ�Ӻ��������ֻ��1�����ӣ����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com