
”¾ĢāÄæ”æA”¢B”¢C”¢DĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĖÄÖÖŌŖĖŲ£¬A2£ŗĶB+¾ßÓŠĻąĶ¬µÄµē×Ó¹¹ŠĶ£»C”¢ DĪŖĶ¬ÖÜĘŚŌŖĖ÷£¬CŗĖĶāµē×Ó×ÜŹżŹĒ×īĶā²ćµē×ÓŹżµÄ3±¶£»DŌŖĖŲ×īĶā²ćÓŠŅ»øöĪ“³É¶Ōµē×Ó”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĖÄÖÖŌŖĖŲÖŠµēøŗŠŌ×īŠ”µÄŹĒ________£ØĢīŌŖĖŲ·ūŗÅ£©£¬ĘäÖŠCŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼Ķ¼ĪŖ________”£
£Ø2£©AŗĶBµÄĒā»ÆĪļĖłŹōµÄ¾§ĢåĄąŠĶ·Ö±šĪŖ_________ŗĶ_________”£
£Ø3£©B”¢C¾łæÉŅŌÓėDŠĪ³É»ÆŗĻĪļ£¬ĘäÖŠČŪµć½ĻøߵďĒ____£ØÓĆ»ÆѧŹ½±ķŹ¾£©
£Ø4£©AŗĶBæÉŠĪ³É1:1ŠĶµÄ»ÆŗĻĪļE£¬EµÄµē×ÓŹ½ĪŖ_____
£Ø5£©»ÆŗĻĪļD2AµÄĮ¢Ģå¹¹ŠĶĪŖ_________£¬ÖŠŠÄŌ×ӵĹĀµē×Ó¶ŌŹżĪŖ_________£¬µ„ÖŹDÓėŹŖČóµÄNa2CO3·“Ó¦æÉÖʱøD2A£¬Ęä»Æѧ·½³ĢŹ½ĪŖ_______________”£
£Ø6£©AŗĶBÄܹ»ŠĪ³É»ÆŗĻĪļF£¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾£¬¾§°ū±ß³¤ĪŖ0.566nm£¬ F µÄ»ÆѧŹ½ĪŖ______£»¾§°ūÖŠA Ō×ÓµÄÅäĪ»ŹżĪŖ______£»¾§ĢåFµÄĆܶČ=______g£®cm£3£ØÖ»ĮŠŹ½£¬²»¼ĘĖć£©

”¾“š°ø”æ£Ø1£©Na£Ø1·Ö£©£» £Ø1·Ö£©![]()
£Ø2£©·Ö×Ó¾§Ģå£Ø1·Ö£© Ąė×Ó¾§Ģå£Ø1·Ö£©
£Ø3£©NaCl
£Ø4£©![]()
£Ø5£©VŠĪ£»2£»
2Cl2£«2Na2CO3£«H2O£½Cl2O£«2NaHCO3£«2NaCl
£Ø6£©Na2O£»8£»![]() £ØÓĆNA±ķŹ¾Ņ²æÉ£©
£ØÓĆNA±ķŹ¾Ņ²æÉ£©
”¾½āĪö”æŹŌĢā·ÖĪö£ŗCŗĖĶāµē×Ó×ÜŹżŹĒ×īĶā²ćµē×ÓŹżµÄ3±¶£¬Ó¦ĪŖPŌŖĖŲ£¬C”¢DĪŖĶ¬ÖÜĘŚŌŖĖŲ£¬ŌņÓ¦ĪŖµŚČżÖÜĘŚŌŖĖŲ£¬DŌŖĖŲ×īĶā²ćÓŠŅ»øöĪ“³É¶Ōµē×Ó£¬Ó¦ĪŖClŌŖĖŲ£¬A2-ŗĶB+¾ßÓŠĻąĶ¬µÄµē×Ó¹¹ŠĶ£¬½įŗĻŌ×ÓŠņŹż¹ŲĻµæÉÖŖAĪŖOŌŖĖŲ£¬BĪŖNaŌŖĖŲ£»
£Ø1£©ĖÄÖÖŌŖĖŲ·Ö±šĪŖO”¢Na”¢P”¢Cl£¬µēøŗŠŌ×ī“óµÄĪŖOŌŖĖŲ£¬CĪŖPŌŖĖŲ£¬ŗĖĶāµē×ÓÅŲ¼ĪŖ1s22s22p63s23p3£¬¼Ūµē×ÓÅŲ¼Ķ¼ĪŖ![]() £»
£»
£Ø2£©AµÄĒā»ÆĪļĪŖĖ®£¬ĪŖ·Ö×Ó¾§Ģ壬BµÄĒā»ÆĪļĪŖNaH£¬ĪŖĄė×Ó¾§Ģ壬
¹Ź“š°øĪŖ£ŗO3£»O3Ļą¶Ō·Ö×ÓÖŹĮæ½Ļ“󣬷¶µĀ»ŖĮ¦½Ļ“ó£»·Ö×Ó¾§Ģ壻Ąė×Ó¾§Ģ壻
£Ø3£©NaŗĶP¾łÄÜŗĶClŠĪ³É»ÆŗĻĪļNaClÓėPCl3£¬ĘäÖŠNaClĪŖĄė×Ó¾§Ģ壬ČŪ·Šµć½Ļøߣ»
£Ø4£©OŗĶNaæÉŠĪ³É1:1ŠĶµÄ»ÆŗĻĪļNa2O2£¬Ęäµē×ÓŹ½ĪŖ![]() £»
£»
£Ø5£©»ÆŗĻĪļD2AĪŖCl2O£¬OĪŖÖŠŠÄŌ×Ó£¬ŠĪ³É2øö¦Ä¼ü£¬¹Āµē×Ó¶ŌŹżĪŖ![]() =2£¬ŌņÖŠŠÄŌ×ӵļŪ²ćµē×Ó¶ŌŹżĪŖ4£¬Į¢Ģå¹¹ŠĶĪŖVŠĪ£¬ĀČĘųÓėŹŖČóµÄNa2CO3·“Ó¦æÉÖʱøCl2O£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl£»
=2£¬ŌņÖŠŠÄŌ×ӵļŪ²ćµē×Ó¶ŌŹżĪŖ4£¬Į¢Ģå¹¹ŠĶĪŖVŠĪ£¬ĀČĘųÓėŹŖČóµÄNa2CO3·“Ó¦æÉÖʱøCl2O£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl£»
£Ø6£©AŗĶBÄܹ»ŠĪ³É»ÆŗĻĪļFĪŖĄė×Ó»ÆŗĻĪļ£¬ŅõĄė×ÓĪ»ÓŚ¾§°ūµÄ¶„µćŗĶĆęŠÄ£¬ŃōĄė×ÓĪ»ÓŚ¾§°ūµÄĢåŠÄ£¬ŌņNaµÄøöŹżĪŖ8£¬OµÄøöŹżĪŖ8”Į+6”Į=4£¬N£ØNa£©£ŗN£ØO£©=2£ŗ1£¬ŌņŠĪ³ÉµÄ»ÆŗĻĪļĪŖNa2O£¬¾§°ūÖŠOĪ»ÓŚ¶„µć£¬NaĪ»ÓŚĢåŠÄ£¬Ćæøö¾§°ūÖŠÓŠ1øöNaÓėOµÄ¾ąĄė×ī½ü£¬Ćæøö¶ØµćĪŖ8øö¾§°ū¹²ÓŠ£¬Ōņ¾§°ūÖŠOŌ×ÓµÄÅäĪ»ŹżĪŖ8£¬¾§°ūµÄÖŹĮæĪŖ![]() £¬¾§°ūµÄĢå»żĪŖ£Ø0.566”Į10-7£©cm3£¬Ōņ¾§ĢåFµÄĆܶČĪŖ
£¬¾§°ūµÄĢå»żĪŖ£Ø0.566”Į10-7£©cm3£¬Ōņ¾§ĢåFµÄĆܶČĪŖ![]() ”£
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æäåŅŅĶéŌŚ²»Ķ¬ČܼĮÖŠÓėNaOH·¢Éś²»Ķ¬ĄąŠĶµÄ·“Ó¦£¬Éś³É²»Ķ¬µÄ·“Ó¦²śĪļ”£Ä³Ķ¬Ń§ŅĄ¾ŻäåŅŅĶéµÄŠŌÖŹ£¬ÓĆĻĀĶ¼ŹµŃé×°ÖĆ(Ģś¼ÜĢØ”¢¾Ę¾«µĘĀŌ)Ńé֤Ȕ“ś·“Ó¦ŗĶĻūČ„·“Ó¦µÄ²śĪļ£¬ĒėÄćŅ»Ęš²ĪÓėĢ½¾æ”£
ŹµŃé²Ł×÷¢ń£ŗŌŚŹŌ¹ÜÖŠ¼ÓČė5 mL 1 mol/L NaOHČÜŅŗŗĶ5 mLäåŅŅĶ飬Õńµ“”£
ŹµŃé²Ł×÷II£ŗ½«ŹŌ¹ÜČēĶ¼¹Ģ¶Øŗó£¬Ė®Ō”¼ÓČČ”£

£Ø1£©ÓĆĖ®Ō”¼ÓČȶų²»Ö±½ÓÓĆ¾Ę¾«µĘ¼ÓČȵÄŌŅņŹĒ________”£
£Ø2£©¹Ū²ģµ½___________ĻÖĻóŹ±£¬±ķĆ÷äåŅŅĶéÓėNaOHČÜŅŗŅŃĶźČ«·“Ó¦”£
£Ø3£©¼ų¶ØÉś³ÉĪļÖŠŅŅ“¼µÄ½į¹¹£¬æÉÓĆµÄ²ØĘ׏Ē_____”£
£Ø4£©ĪŖÖ¤Ć÷äåŅŅĶéŌŚNaOHŅŅ“¼ČÜŅŗÖŠ·¢ÉśµÄŹĒĻūČ„·“Ó¦£¬ŌŚÄćÉč¼ĘµÄŹµŃé·½°øÖŠ£¬ŠčŅŖ¼ģŃéµÄŹĒ____£¬¼ģŃéµÄ·½·ØŹĒ (ŠčĖµĆ÷£ŗĖłÓƵďŌ¼Į”¢¼ņµ„µÄŹµŃé²Ł×÷¼°Ō¤²ā²śÉśµÄŹµŃéĻÖĻó)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¢ń.ijŹµŃ銔×é¶ŌH2O2µÄ·Ö½ā×öĮĖČēĻĀĢ½¾æ”£ĻĀ±ķŹĒøĆŹµŃ銔×éŃŠ¾æÓ°ĻģH2O2·Ö½āĖŁĀŹµÄŅņĖŲŹ±¼ĒĀ¼µÄŅ»×鏿¾Ż£¬½«ÖŹĮæĻąĶ¬µ«×“Ģ¬²»Ķ¬µÄMnO2·Ö±š¼ÓČėŹ¢ÓŠ15 ml 5%µÄH2O2ČÜŅŗµÄ“óŹŌ¹ÜÖŠ£¬²¢ÓĆ“ų»šŠĒµÄľĢõ²āŹŌ£¬½į¹ūČēĻĀ£ŗ

£Ø1£©Š“³öÉĻŹöŹµŃéÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŹµŃé½į¹ū±ķĆ÷£¬“߻ƼĮµÄ“߻Ɗ§¹ūÓė ÓŠ¹Ų”£
¢ņ. ¾ŃŠ¾æÖŖCu2+¶ŌH2O2·Ö½āŅ²¾ßÓŠ“ß»Æ×÷ÓĆ£¬ĪŖ±Č½ĻFe3+ŗĶCu2+¶ŌH2O2·Ö½āµÄ“߻Ɗ§¹ū£¬Ä³ŃŠ¾æŠ”×éµÄĶ¬Ń§·Ö±šÉč¼ĘĮĖČēĶ¼¼×”¢ŅŅĖłŹ¾µÄŹµŃ锣»Ų“šĻą¹ŲĪŹĢā£ŗ
£Ø3£©¶ØŠŌ·ÖĪö£ŗČēĶ¼¼×æÉĶعż¹Ū²ģ £¬¶ØŠŌ±Č½ĻµĆ³ö½įĀŪ”£ÓŠĶ¬Ń§Ģį³ö½«FeCl3øÄĪŖFe2(SO4)3øüĪŖŗĻĄķ£¬ĘäĄķÓÉŹĒ ”£

£Ø4£©¶ØĮæ·ÖĪö£ŗČēĶ¼ŅŅĖłŹ¾£¬ŹµŃ鏱¾łŅŌÉś³É40 mLĘųĢåĪŖ×¼£¬ĘäĖūæÉÄÜÓ°ĻģŹµŃéµÄŅņĖŲ¾łŅŃŗöĀŌ”£Ķ¼ÖŠŅĒĘ÷AµÄĆū³ĘĪŖ £¬ŹµŃéÖŠŠčŅŖ²āĮæµÄŹż¾ŻŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠ»ś»ÆŗĻĪļGŹĒŗĻ³ÉĪ¬ÉśĖŲĄąŅ©ĪļµÄÖŠ¼äĢ壬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗ

ĘäÖŠA”«F·Ö±š“ś±ķŅ»ÖÖÓŠ»ś»ÆŗĻĪļ£¬ŗĻ³ÉĀ·ĻßÖŠ²æ·Ö²śĪļ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„ŅŃÖŖ£ŗ

GĪŖ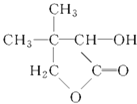 £»
£»
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©GµÄ·Ö×ÓŹ½_____________£»DÖŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ_________”£
£Ø2£©µŚ¢Ś²½·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________”£
£Ø3£©µŚ¢Ū²½·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________”£
£Ø4£©Š“³öFµÄ½į¹¹¼ņŹ½_____________”£
£Ø5£©µŚ¢Ł~¢Ž²½·“Ó¦ÖŠŹōÓŚ¼Ó³É·“Ó¦µÄÓŠ___________________£»ŹōÓŚČ”“ś·“Ó¦µÄÓŠ
__________________________”££ØĢī²½Ö豹ŗÅ£©
£Ø6£©Ķ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄEµÄĶ¬·ÖŅģ¹¹ĢåÓŠ_____________ÖÖ”£
¢ŁÖ»ŗ¬Ņ»ÖÖ¹ŁÄÜĶÅ£»
¢ŚĮ“ד½į¹¹ĒŅĪŽ”ŖO”ŖO”Ŗ£»
¢ŪŗĖ“Ź²ÕńĒāĘ×Ö»ÓŠ2×é·å”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖ·“Ó¦X+YØTM+NĪŖ·ÅČČ·“Ó¦£¬¶ŌøĆ·“Ó¦µÄĖµ·ØÕżČ·ŹĒ
A. XµÄÄÜĮæŅ»¶ØøßÓŚM
B. YµÄÄÜĮæŅ»¶ØøßÓŚN
C. XŗĶYµÄ×ÜÄÜĮæŅ»¶ØøßÓŚMŗĶNµÄ×ÜÄÜĮæ
D. ŅņĪŖøĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬¹Ź²»±Ų¼ÓČČ¾ĶæÉ·¢Éś
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆĻĀĶ¼×°ÖĆ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ

¢ŁĮæČ”50mL 0.25mol/L H2SO4ČÜŅŗµ¹ČėŠ”ÉÕ±ÖŠ£¬²āĮæĪĀ¶Č£»
¢ŚĮæČ”50mL 0.55mol/L NaOHČÜŅŗ£¬²āĮæĪĀ¶Č£»
¢Ū½«NaOHČÜŅŗµ¹ČėŠ”ÉÕ±ÖŠ,»ģŗĻ¾łŌČŗó²āĮæ»ģŗĻŅŗĪĀ¶Č”£Ēė»Ų“š£ŗ
£Ø1£©ČēÓŅĶ¼ĖłŹ¾£¬ŅĒĘ÷AµÄĆū³ĘŹĒ_________ ______£»
£Ø2£©NaOHČÜŅŗÉŌ¹żĮæµÄŌŅņ ____________________________”£
£Ø3£©¼ÓČėNaOHČÜŅŗµÄÕżČ·²Ł×÷ŹĒ_______£ØĢī×ÖÄø£©”£
A£®ŃŲ²£Į§°ō»ŗĀż¼ÓČė B£®Ņ»“ĪŃøĖŁ¼ÓČė C£®·ÖČż“Ī¼ÓČė
£Ø4£©Ź¹ĮņĖįÓėNaOHČÜŅŗ»ģŗĻ¾łŌȵÄÕżČ·²Ł×÷ŹĒ ________ __________________”£
£Ø5£©ÉčČÜŅŗµÄĆÜ¶Č¾łĪŖ1g”¤cm-3,ÖŠŗĶŗóČÜŅŗµÄ±ČČČČŻc=4.18 J”¤£Øg”¤”ę£©-1£¬Ēėøł¾ŻŹµŃ鏿¾ŻŠ“³öøĆÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½_______________________________

£Ø6£©ÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£©
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
c£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
£Ø7£©ŌõŃł²ÅÄÜČ·±£¶ĮČ”»ģŗĻŅŗµÄ×īøßĪĀ¶Č£æ_______ ____________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÓŠ¹ŲĪļÖŹµÄŠŌÖŹ»ņÓ¦ÓƵÄĖµ·ØÕżČ·µÄŹĒ
A£®¾§Ģå¹čŹĒ¹āĻĖÖĘĘ·µÄÖ÷ŅŖ»Æѧ³É·Ö B£®ŗĻ½šÖĮÉŁŗ¬Į½ÖÖŅŌÉĻµÄ½šŹōŌŖĖŲ
C£®½ŗĢåæɲśÉś¶”“ļ¶ūŠ§Ó¦ D£®ŹÆÓĶ·ÖĮóæÉ»ńµĆŅŅĻ©”¢±ūĶéŗĶ±ūĻ©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµē½ā·Ø“¦ĄķµŖŃõ»ÆĪļ·ĻĘųÓŠ½ĻøߵĻ·¾³Š§ŅęŗĶ¾¼ĆŠ§Ņę£ØĶ¼ÖŠµē¼«¾łĪŖŹÆÄ«£©”£


£Ø1£©µē½āNOÖʱøNH4NO3ŌĄķČē×óĶ¼ĖłŹ¾£ŗ
¢ŁŃō¼«ĪŖ_______ (ĢīX»ņY)£¬YµÄµē¼«·“Ó¦Ź½ĪŖ________________________________”£
¢ŚĪŖŹ¹µē½ā²śĪļĶźČ«×Ŗ»ÆĪŖNH4NO3£¬ŠčŅŖ²¹³äµÄĪļÖŹAµÄ»ÆѧŹ½ĪŖ________________”£
£Ø2£©ÓĆČēĶ¼×°ÖĆ½ųŠŠÄ£Äāµē½āNO2ĘųĢåŹµŃ飬æÉ»ŲŹÕĻõĖį”£
¢Łµē½āŹ±NO2·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½_________________________________”£
¢ŚČōÓŠ±ź×¼×“æöĻĀ2.24 LNO2±»ĪüŹÕ£¬ĶعżŃōĄė×Ó½»»»Ä¤£ØÖ»ŌŹŠķŃōĄė×ÓĶعż£©µÄH+ĪŖ_________________mol”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æīŃŹĒŗ½æÕ”¢¾ü¹¤”¢µēĮ¦µČĮģÓņµÄÖŲŅŖŌĮĻ”£¹¤ŅµÉĻÓĆīŃĖįŃĒĢś£ØFeTiO3£©Ņ±Į¶īŃ£ØTi£©µÄ¹ż³ĢŹĒ£ŗ¢Ł2FeTiO3£«6C£«7Cl2![]() 2TiCl4£«2FeCl3£«6CO
2TiCl4£«2FeCl3£«6CO
¢ŚŌŚė²Ęų»·¾³ÖŠ£¬2Mg£«TiCl4![]() Ti£«2MgCl2
Ti£«2MgCl2
ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ£Ø £©
A£®·“Ó¦¢ŚŹōÓŚÖĆ»»·“Ó¦
B£®·“Ó¦¢ŚÖŠĀČ»ÆĪļµÄ×ÜÖŹĮæ±£³Ö²»±ä
C£®·“Ó¦¢Ł”¢¢ŚÖŠīŃŌŖĖŲµÄ»ÆŗĻ¼Ū¶¼øıä
D£®·“Ó¦¢ŚÖŠ£¬ė²ĘųÖ»×÷ĪŖ±£»¤Ęų²¢²»²Ī¼Ó·“Ó¦
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com