

| n |
| V |
| n |
| V |
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������HCl��Һ�����֮���ϵ��ͼ��ʾ��ͨ������ش�
ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������HCl��Һ�����֮���ϵ��ͼ��ʾ��ͨ������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ұ���ղ��������紵���� |
| B�����ϵ���˿����������ɻ���ʼ�� |
| C��ֻҪ���������ĥ���� |
| D������һ�������꣬������ů������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0.1 mol/LCH3COONa��Һ��c(OH-)=c(CH3COOH)+c(H+) |
| B����0.1 mol/L��ˮ�м�����������粒��壬��ҺpH��С |
| C����ˮ�еμ��ռ���Һ����c��Cl-��+c��ClO-��=c��Na+��ʱ����Һһ���dz����� |
| D��ij�¶��½�ϡ��ˮ�μӵ�ϡ�����У�����Һ��pH=7ʱ��һ����c(Cl-)=c(NH4+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
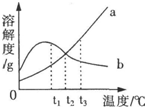 �й�a��b�������ʵ��ܽ��������ͼ��ʾ��������������ȷ���ǣ�������
�й�a��b�������ʵ��ܽ��������ͼ��ʾ��������������ȷ���ǣ�������| A��a���ʵ��ܽ�������¶ȵ����߶����� |
| B����t2��ʱ��a��b�������ʵ���Һ�����ʵ���������һ����� |
| C��t3��ʱ��a���ʵ��ܽ�ȴ���b���ʵ��ܽ�� |
| D����a��b�������ʵı�����Һ��t3�潵����t1�棬a�о���������b�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��6.5g����п���п����ʱʧȥ�ĵ�����ĿΪ0.1NA |
| B�����³�ѹ�£�2g��������ԭ����ĿΪ2NA |
| C����״���£�11.2LH2O���еķ�����Ϊ0.5NA |
| D�����³�ѹ�£�11.2LCl2���еķ�����Ϊ0.5NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com