
����P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ����ͼ�еġ�H��ʾ����1mol��������ݣ���
��1��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ_____________________________________��
��2��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ______________________________________��
��3��P��Cl2��������Ӧ����1molPCl5��
��H3��_________��P��Cl2һ����Ӧ����1molPCl5�ġ�H4__________��H3 (����ڡ�����С�ڡ����ڡ�)��
��4��PCl5������ˮ��ַ�Ӧ���������������ᣬ�仯ѧ����ʽ��__________________________��
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ӧ���������ʲ�����ͬʱ�������������仯��
(1)��һ��С�ձ������Լ20 g����ĥ�ɷ�ĩ��������������[Ba(OH)2·8H2O]����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ���ټ���Լ10 g NH4Cl���壬�������ò�����Ѹ�ٽ���(����ͼ)��

��ش��������⣺
�ٲ����У������ò�����Ѹ�ٽ����Ŀ����______________��
�ڷ�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
�۹۲쵽������(�д̼�����ζ����)��_____________________ _________���������˵����������__________________________��
(2)ij��ѧС�黹�����һ��ʵ��(��ͼ)��

����֬�ް�סԼ0.2 g Na2O2��ĩ������ʯ�����ϡ�����֬���ϵμӼ���ˮ��
��ش��������⣺
�ٹ���������ˮ������Ӧ�Ļ�ѧ����ʽΪ_______________________________________________________��
�ڹ۲쵽��������__________________������������������ԭ��_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ������ﲻ��ȷ����
A. ������ĵ���ʽ��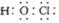 B. ������Ϊ6��������Ϊ14��ԭ�ӣ�
B. ������Ϊ6��������Ϊ14��ԭ�ӣ�
C.CS2�Ľṹʽ�� D. �ȵ�ԭ�ӽṹʾ��ͼ��
D. �ȵ�ԭ�ӽṹʾ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ������п�ۺ�6mol��L-1�Ĺ������ᷴӦ���������м�����������������ʱ���ܹ��ӿ췴Ӧ���ʣ��ֲ�Ӱ�����H2�������ǣ� ��
��ʯī ��CuO ��ͭ�� ������ ��Ũ���� ����ˮ����
A.�٢ۢ� B.�٢ۢ� C.�ڢܢ� D.�ڢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������·�Ӧ��X2(g)+Y2(g)  2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п����� �� ��
2Z(g)����֪X2��Y2��Z����ʼŨ�ȷֱ�Ϊ0.1mol/L��0.3mol/L��0.2mol/L����һ�������£�����Ӧ�ﵽƽ��ʱ�������ʵ�Ũ���п����� �� ��
A��X2Ϊ0.2mol/L B��Y2Ϊ0.4mol/L C�� ZΪ0.3mol/L D�� ZΪ0.4mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����
A.��״���£�11.2 L Cl2ͨ�뺬0.5molFeBr2����Һ��ת�Ƶ�����Ϊ1.5NA
B.0.1 mol��L��1��AlCl3��Һ������NaOH��Һ��Ӧ���ò����к�Al Ϊ0.1NA
Ϊ0.1NA
C.����Zn��Ũ���Ṳ�ȿ����ɱ�״���µ�����2.24 L����μӷ�Ӧ������Ϊ0.4 NA
D.���³�ѹ�£�5.6g������;���ϩ�Ļ�����к��е�̼ԭ����Ϊ0.4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E���ֳ���������������±��е������γɵģ�
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ |
|
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ������BΪ��ɫ(����ɫ�ܲ���)��
���ڸ���Һ�м��������ữ�����ᱵ�� Һ��ֻ
Һ��ֻ ��A�зų���ɫ���壬ֻ��D�в�����ɫ������
��A�зų���ɫ���壬ֻ��D�в�����ɫ������
�ݽ�B��C����Һ��ϣ�δ���������������ɡ���������ʵ����գ�
(1)д��B��D�Ļ�ѧʽ��B ___��D ��
(2)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��______________��
(3)����0.01 mol A����Һ�뺬0.02 mol E����Һ��Ӧ��, ����Һ�еμ�0.1 mol��L��1ϡ���ᡣ����ͼ������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵ����


(4)��m mL b mol��L��1 C��Һ�У���������a mol��L��1 ��E��Һ��
��a��3bʱ�����ɳ��������ʵ����� mol��
��3b��a��4bʱ�����ɳ��������ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������������Ļ���������к�̼Ԫ�ص���������Ϊx����������������������Ϊ(����)
A. B��1��
B��1��
C��1�� x D��������
x D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾΪ����һ�ֹǼ���ʽ������Ϊ��λ��ռ����죬��ô�ñ��е�ÿ��ˮ�����������Ϊ(����)

A��1 B��3
C��2 D��4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com