
����Ŀ��������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4+��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԣ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��֪����Ag+��Һ�еμӰ�ˮ���Ȳ��������������μӰ�ˮ�������ܽ⡣
��ش��������⣺
��1���������У�һ��û�е���������_____��������������ͬ�������εĻ�ѧʽ��________��
��2��D�Ļ�ѧʽΪ____________��D��Һ�Լ��Ե�ԭ����(�����ӷ���ʽ��ʾ)_____________��
��3��A��C����Һ��Ӧ�����ӷ���ʽ��_______________��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��_______________��
��4����Ҫ����B�������������ӣ���ȷ��ʵ�鷽���ǣ�_________________________��
���𰸡�Cu2����Fe3��(NH4)2SO4��Al2(SO4)3Na2CO3CO32-+H2O![]() HCO3-+OH-Ag����Cl��=AgCl��Al3����3NH3��H2O=Al(OH)3����3NH4+ȡ����B���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲��Ƿ����ɫ
HCO3-+OH-Ag����Cl��=AgCl��Al3����3NH3��H2O=Al(OH)3����3NH4+ȡ����B���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲��Ƿ����ɫ
��������
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ����������Һ��һ������Cu2+��Fe3+��Ba2+��SO42-��CO32-����ͬһ��Һ�У�Al3+��CO32-����ͬһ��Һ�У�Ag+��Cl-��SO42-��CO32-����ͬһ��Һ�У�
��D����ɫ��Ӧ�ʻ�ɫ��D��������ΪNa+��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�B��C��E����Һ�к�NH4+��Al3+��Ag+��D����Һ�ʼ��ԣ�D�к�CO32-�������֪DΪNa2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ������������A��C��Һ�в���CO32-��SO42-��
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ����������֪����Ag+��Һ�еμӰ�ˮ���Ȳ��������������μӰ�ˮ�������ܽ�����C��������ΪAg+��E��������ΪAl3+��Ag+��Cl-��SO42-��CO32-����Һ�в��ܴ���������CΪAgNO3��
����A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����CΪAgNO3��A��������ΪCl-��E��������ΪAl3+��A����Һ�����ԣ�AΪBaCl2��EΪAl2��SO4��3�����ǰ��ķ�����B��Һ�����ԣ���BΪ��NH4��2SO4��
�ݴ˷�������
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ����������Һ��һ������Cu2+��Fe3+��Ba2+��SO42-��CO32-����ͬһ��Һ�У�Al3+��CO32-����ͬһ��Һ�У�Ag+��Cl-��SO42-��CO32-����ͬһ��Һ�У�
��D����ɫ��Ӧ�ʻ�ɫ��D��������ΪNa+��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�B��C��E����Һ�к�NH4+��Al3+��Ag+��D����Һ�ʼ��ԣ�D�к�CO32-�������֪DΪNa2CO3��
�������������ε���Һ�зֱ����Ba��NO3��2��Һ��ֻ��A��C����Һ������������A��C��Һ�в���CO32-��SO42-��
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ����������֪����Ag+��Һ�еμӰ�ˮ���Ȳ��������������μӰ�ˮ�������ܽ�����C��������ΪAg+��E��������ΪAl3+��Ag+��Cl-��SO42-��CO32-����Һ�в��ܴ���������CΪAgNO3��
����A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����CΪAgNO3��A��������ΪCl-��E��������ΪAl3+��A����Һ�����ԣ�AΪBaCl2��EΪAl2��SO4��3�����ǰ��ķ�����B��Һ�����ԣ���BΪ��NH4��2SO4��
��1������������һ��û�е���������Cu2+��Fe3+��������������ͬ�������εĻ�ѧʽ�ǣ�NH4��2SO4��Al2��SO4��3�����������Ӷ�ΪSO42-��
��2��D�Ļ�ѧʽΪNa2CO3��Na2CO3��Һ�ʼ��Ե�ԭ����CO32-������ˮ����CO32-ˮ������ӷ���ʽΪCO32-+H2O![]() HCO3-+OH-��
HCO3-+OH-��
��3��AΪBaCl2��CΪAgNO3��A��C����Һ��Ӧ�����ӷ���ʽΪAg++Cl-=AgCl����EΪAl2��SO4��3��E�백ˮ��Ӧ�����ӷ���ʽΪAl3++3NH3��H2O=Al��OH��3��+3NH4+��
��4��BΪ��NH4��2SO4��B��������ΪNH4+������NH4+����ȷ��ʵ�鷽���ǣ�ȡ����B���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲��Ƿ����ɫ������ֽ����ɫ��˵��B�к�NH4+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ӵ���������ȷ����( )
A. PH3�ĵ���ʽΪ![]() �������ȶ��Բ���NH3
�������ȶ��Բ���NH3
B. HS���ĵ���ʽΪ![]() ���Ǻ��м��Լ���18���ӵ�����
���Ǻ��м��Լ���18���ӵ�����
C. CH2Cl2�ĵ���ʽΪ![]() ���ǽṹ����������ķ���
���ǽṹ����������ķ���
D. KF�ĵ���ʽΪ![]() ������������ˮ�����ӻ�����
������������ˮ�����ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.1 mol��L��1CuSO4��Һ480 mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����Ͳ����������________��________�Լ�����������Ƭ��ֽ��
��2�����㣬Ӧѡ������________��
A����ҪCuSO4����8 g B����ҪCuSO4��5H2O����12.0 g
C����ҪCuSO4��5H2O����12.5 g D����ҪCuSO4����7.7 g
��3����������������������������Һ��Ũ�Ȼ�________(����ƫ������ƫ����������Ӱ����)��
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����____________��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ��________��������Ҫϴ���ձ�2��3����Ϊ��________��
��6�����ݣ�ҡ�ȡ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�����ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
��8�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�________(����ƫ������ƫ����������Ӱ����)��
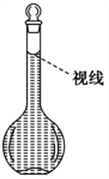
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��������������������ܲ��ɷ֣�����˵����ȷ���ǣ� ��
A.���þ����������̫���ܵ�ؽ�̫����ֱ��ת��Ϊ����
B.��չ������ҵ������˽�˹�����������߳���Ч�ʣ�Ӧ�ô����ᳫ
C.ũ�����ո�����µĽոѿ��Ծ͵ط���
D.������ķ�Һ����ֱ���ŷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ,�����й�NA����������ȷ����( )
����״����,20 g��ˮ(D2O)�к��еĵ�����Ϊ10NA
��0.5 mol Fe2+��������H2O2��Һ��Ӧ,ת��NA������
����2 mol NO��1 mol O2��Ϻ�,��ϵ�еķ�������Ϊ3NA
��������,0.4 mol SiO2�����Ĺ��ۼ���ĿΪ1.6NA
��2 mol��L-1̼������Һ��Na+����Ŀ��2NA
��1 mol�����ܽ���ˮ�еõ���������ˮ�������ӵ���Ŀ��NA
��22.4 L��N2�Ĺ��õ��Ӷ���Ϊ3NA
A. 2�� B. 3�� C. 4�� D. 5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����жԸ�����Ʒ��ȡ�Ĵ�ʩ������ֹ�������ʴ���ǣ� ��
A.����Ϳ����B.������Ƕ����C.������Ƕп��D.���Դ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ⶨ0.1 mol��L-1 Na2SO3��Һ�������ٽ��¹����е�pH���������¡�
ʱ�� | �� | �� | �� | �� |
�¶�/�� | 25 | 30 | 40 | 25 |
pH | 9.66 | 9.52 | 9.37 | 9.25 |
ʵ������У�ȡ�٢�ʱ�̵���Һ�����������ữ��BaCl2��Һ���Ա�ʵ�飬�ܲ�����ɫ�����ࡣ
����˵������ȷ����
A. Na2SO3��Һ�д���ˮ��ƽ����![]() +H2O
+H2O![]()
![]() +OH
+OH
B. ����pH������ͬ��������![]() Ũ�ȼ�С��ɵ�
Ũ�ȼ�С��ɵ�
C. �١����Ĺ��������¶Ⱥ�Ũ�ȶ�ˮ��ƽ���ƶ������Ӱ��һ��
D. ��������Kwֵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����顢��ϩ���Ҵ�������Ҫ���л��������ش��������⣺
��1�������������ĵ�һ��ȡ����Ӧ�Ļ�ѧ����ʽΪ_______��
��2������ϩͨ�뵽������Ȼ�̼��Һ�У���������ã��۲쵽������Ȼ�̼��Һ��ɫ��д���ù����з�����Ӧ�Ļ�ѧ����ʽ_______��
��3�����м��������У���Ϊͬ���칹�����____����Ϊͬϵ�����____��ͬ�����������_____��
A�������������� B�������ͳ��� C��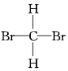 ��
��
D��35Cl��37Cl E��CH3CH2OH��CH3OCH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A��B��C��D��E���ֿ�����ǿ����ʣ�������ˮ�пɵ�������������ӣ�������H+��Na+��Al3+��Ag+��Ba2+��������OH-��Cl-��CO32-��NO3-��SO42-(�������Ӳ��ظ�)����֪����A��B����Һ�ʼ��ԣ�C��D��E��Һ�����ԡ�����E��Һ����εμ�B��Һ�������������������Ӻ���ٵ�����ʧ����D��Һ������������Һ��Ӧ���ܲ����������Իش���������:
(1)д��A��D�Ļ�ѧʽ:A___________D___________________��
(2)A��E��Һ��Ӧ�����ӷ���ʽ_________________________��
(3)��֪:H+(aq)+OH-(aq)=H2O(l)��H=-akJ/mol����д����ͬ������B��C��ϡ��Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ_________________________________��
(4)����E��Һ�������ӵķ���Ϊ__________________________��
(5)��д����E��Һ�мӹ���B��Һ�Ļ�ѧ����ʽ_____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com