
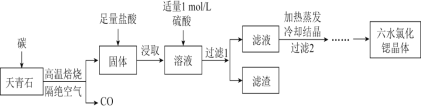
����Ŀ����(Sr)Ϊ��������IIA��Ԫ�أ��仯������ˮ�Ȼ���(SrCl26H2O)��ʵ������Ҫ�ķ����Լ�����ҵ�ϳ�������ʯ(��Ҫ�ɷ�ΪSrSO4)Ϊԭ���Ʊ�������������ͼ��
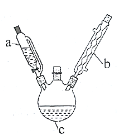
��֪���پ������ȡ�����Һ�г�����Sr2+��Cl-�⣬����������Ba2+���ʡ�
��BaSO4���ܶȻ�����Ϊ2.2��10-10��SrSO4���ܶȻ�����Ϊ3.3��10-7��
��SrCl26H2O��Ħ������Ϊ267g/mol��
(1)��ҵ������ʯ����ǰӦ����ĥ���飬��Ŀ����__��
(2)��ҵ������ʯ�����������±���ʱ����0.5molSrSO4��ֻ��SԪ�ر���ԭ����ת����4mol���ӡ���÷�Ӧ�Ļ�ѧ����ʽΪ__��
(3)��ȡ����������Ŀ����___�������ӷ���ʽ��ʾ___��
(4)��Ʒ���ȼ�⣺��ȡ2.000g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO30.01mol��AgNO3��Һ����Һ�г�Cl-�⣬����������Ag+��Ӧ�����ӣ���Cl-��ȫ��������1��2�κ�Fe3+����Һ��ָʾ������0.2000mol/L��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag+��AgSCN��ɫ��������ʽ��������֪��SCN-����Ag+��Ӧ��
�ٵζ���Ӧ�ﵽ�յ��������__��
�����ζ�������ȥ����Ũ�ȵ�NH4SCN��Һ20.00mL�����Ʒ��SrCl26H2O�������ٷֺ���Ϊ___(���г�����ʽ���������)��
(5)��SrCl26H2O������ȡ��ˮ�Ȼ��ȵ���Ҫ�������˾ƾ��ơ������ǡ����ż��⣬����___��
���𰸡����ӷ�Ӧ��ĽӴ���������ѧ��Ӧ���� SrSO4+4C![]() SrS+4CO�� ��ȥ����Ba2+ SO
SrS+4CO�� ��ȥ����Ba2+ SO![]() +Ba2+=BaSO4�� ���������1��NH4SCN��Һʱ����Һ����ɫ��Ϊ��ɫ����30s�ڲ���ɫ
+Ba2+=BaSO4�� ���������1��NH4SCN��Һʱ����Һ����ɫ��Ϊ��ɫ����30s�ڲ���ɫ ![]() ��100% ����
��100% ����
��������
������ʯ(��Ҫ�ɷ�ΪSrSO4)Ϊԭ���Ʊ���ˮ�Ȼ���(SrCl26H2O)�������̿�֪������ʯ��̼�����������±�������CO��SrS��SrS�������ܽ��������Һ�г�����Sr2+��Cl-�⣬����������Ba2+���ʣ�Ȼ��������������ᱵ���������Թ��˺�����Ϊ���ᱵ����Һ����Ҫ��SrCl2����������������ȴ�ᾧ�����˵õ�SrCl26H2O���ݴ˷������
(1)��ĥ�����Ŀ�������ӷ�Ӧ��ĽӴ���������ѧ��Ӧ���ʣ�
(2)��0.5molSrSO4��ֻ��S����ԭ��ת����4mol���ӣ���1mol SrSO4��Ӧ����ת��8mol�����������ȵĻ�ԭ����ΪSrS����÷�Ӧ�Ļ�ѧ����ʽΪSrSO4+4C![]() SrS+4CO����
SrS+4CO����
(3)���ᱵ���ܶȻ������������ȵ��ܶȻ�����С�ö࣬����HCl�ܽ�SrS�����Һ�м��������Ŀ���dz�ȥ��Һ��Ba2+���ʣ���ӦΪ��SO42-+Ba2+=BaSO4����
(4)����֪��SCN-����Ag+��Ӧ��NH4SCN��ʣ���Ag+����γ�AgSCN��ɫ������������Һ�е�Ag+ȫ���������ټ���NH4SCN�������SCN-�ͻ���Fe3+���������ʹ��Һ��Ϊ��ɫ����˵��������1��NH4SCN��Һ�ﵽ�յ�ʱ����Һ����ɫ��Ϊ��ɫ����30 s����ɫ��
��n(NH4SCN)=0.2000mol/L��0.02L=0.2��0.02mol��Ag+��AgSCN��ɫ��������ʽ������������Һ�й�����Ag+�����ʵ���Ϊ��n(Ag+)=0.2��0.02mol������Cl-��Ӧ��Ag+�����ʵ���n(Ag+)=0.01mol-0.2��0.02mol =(0.01-0.2��0.02)mol��2.000g��Ʒ��SrCl26H2O�����ʵ���n(SrCl26H2O)=![]() ��n(Ag+)=
��n(Ag+)=![]() mol��2.000g��Ʒ��SrCl26H2O������m(SrCl26H2O)=
mol��2.000g��Ʒ��SrCl26H2O������m(SrCl26H2O)=![]() mol��267 g/mol=
mol��267 g/mol=![]() g�����Բ�Ʒ����Ϊ��
g�����Բ�Ʒ����Ϊ�� ��100%=
��100%=![]() ��100%��
��100%��
(5)��SrCl26H2O������ȡ��ˮ�Ȼ��ȵ������оƾ��ơ������ǡ����żܡ�������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ϳ�Τ(Abacavir)��һ�ֺ�������ת¼ø���Ƽ������ڿ�������Ч��������ϳ��м���M��![]() ��������˵����ȷ���ǣ� ��
��������˵����ȷ���ǣ� ��
A.�뻷�촼��Ϊͬϵ��
B.����������̼ԭ�ӹ�ƽ��
C.��ʹ���Ը��������Һ����ˮ��ɫ���ҷ�Ӧ������ͬ
D.����̼������Һ���������M
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ��е�λ����ͼ��ʾ����֪Yԭ�ӵ������������Ǵ�����������3��������˵������ȷ����(����)
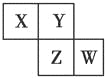
A. ԭ�Ӱ뾶��W��Z��Y��X
B. ����������Ӧˮ��������ԣ�Z��W��X
C. �⻯����ȶ��ԣ�X��Y��Z
D. ����Ԫ�صĵ����У�Z���ʵ��ۡ��е����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Mg�CAgCl�����һ���Ժ�ˮΪ�������Һ��ˮ�����ء���ͼ�ø�ˮ������Ϊ��Դ���NaCl��Һ��ʵ���У�X�缫������ɫ�����ݳ��������йط�����ȷ����

A. IIΪ�������䷴ӦʽΪAg+ + e�C =Ag
B. ˮ��������Cl�C��������Ǩ��
C. ÿת��1 mole-��U��������0. 5mol H2O
D. ��ʼʱU����Y������pH������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ���仯�����ڿ�ѧ�о���ҵ�����о���������;����ش��������⣺
��1��������̬Cuԭ�ӵļ۵����Ų�ͼ__________________��
��2����֪������Cu2O��CuO�ȶ����Ӻ�������Ų��ǶȽ�������Cu2O���ȶ���ԭ��_________________________________________________________________________��
��3�������[Cu(NH3)2]OOCCH3��̼ԭ�ӵ��ӻ�������____________���������ṩ�¶Ե��ӵ�ԭ����____________��C��N��O��Ԫ�صĵ�һ�������ɴ�С��˳����__________����Ԫ�ط��ű�ʾ����
��4��ͭ������ͭԭ�ӵĶѻ���ʽ��ͼ1��ʾ������ͭԭ�ӵĶѻ���ʽΪ________________��
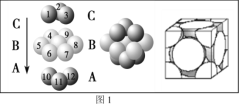
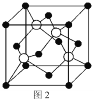
��5��Mԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ2��ʾ���������ͭԭ�ӣ���
�ٸþ���Ļ�ѧʽΪ_________________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������_________���������ӡ��������ۡ���
����֪�þ�����ܶ�Ϊ![]() g/cm3�������ӵ�������ֵΪNA����þ�����Cuԭ�Ӻ�Mԭ��֮�����̾���Ϊ_________pm��д������ʽ����
g/cm3�������ӵ�������ֵΪNA����þ�����Cuԭ�Ӻ�Mԭ��֮�����̾���Ϊ_________pm��д������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪±��һ�������¿��Եõ�ϩ������ͨ�����²�����![]() ��ȡ
��ȡ![]() ����ϳ��������£�
����ϳ��������£�![]()
![]()
��֪����A�Ľṹ��ʽΪ![]()
��ش��������⣺
��1��A��B��Ӧ������________ A�����������ŵ�����Ϊ________
��2��B�Ľṹ��ʽΪ___________________��
��3��A�D��B������Լ��ͷ�Ӧ����Ϊ__________��
��4��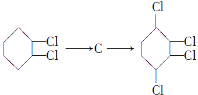 ��������Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ__________________��
��������Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ����ȡ������������(�ƾ��Ƶ���ͼ�о�����ȥ)������գ�
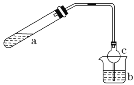
(1)�Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2 mL����ȷ�ļ���˳������______��
(2)Ϊ��ֹa�е�Һ����ʵ��ʱ�������У��ڼ���ǰӦ��ȡ�Ĵ�ʩ��_____________________��
(3)ʵ���м����Թ�a��Ŀ���ǣ�
��_____________________________________________________��
��______________________________________________________��
a�з�Ӧ�Ļ�ѧ����ʽ��__________________________________��
(4)���θ����c��������_________________________________�� b�ձ��м��б���Na2CO3��Һ����������_____________________��
(5)����Ӧǰ��b�м��뼸�η�̪����Һ�ʺ�ɫ����Ӧ������b�е�������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ⱥ����ܱ�������ͨ��SO2��NO2����һ��������ʹ��ӦSO2(g)��NO2(g)![]() SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ������ʾ����ͼ�ɵó�����ȷ������( )
SO3(g)��NO(g)�ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ������ʾ����ͼ�ɵó�����ȷ������( )
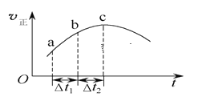
A.��Ӧ��c��ﵽƽ��״̬
B.��Ӧ��Ũ�ȣ�a��С��b��
C.��Ӧ��������������������������
D.��t1����t2ʱ��SO2��ת���ʣ�a��b��С��b��c��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�����ͼ��ʾ��ʵ��װ�ã�ͨ��������Ӧ������CO2��H2O����������ȷ���û�����и���ֵ�����������
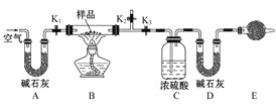
(1)ʵ�鲽�裺
�ٰ�ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����__________��
�ڳ�ȡ��Ʒ�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC��������װ��ʯ�ҵ�U�ι�D��������
�۴���K1��K2���ر�K3������������������ӣ���Ŀ����________��
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ________��________��
�ݴ���K1������������������ӣ�Ȼ�����װ�ã��ٴγ���ϴ��ƿC��������U�ι�D��������
(2)���ڸ�ʵ�鷽������ش��������⡣
�������ȷ�Ӧ����������Բⶨ�����Ӱ����_______________��
��E���������ʢ�ŵ�ҩƷ�Ǽ�ʯ�ң���������_____________�����ʵ����û�и�װ�ã���ᵼ�²������NaHCO3������_____________(����ƫ������ƫС��������Ӱ����)��
������Ʒ����Ϊw g����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1g��m2g����������Na2CO3��10H2O����������Ϊ________(�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com