
��16�֣�50mL 0.50mol/L�����50mL 0.55 mol/L NaOH��Һ����ͼ��ʾ��װ�� �н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȡ�
(1)�ձ���������ĭ���ϵ������� ��
(2)���ձ���������Ӳֽ�壬��õ��к�����ֵ ���ƫ�� ��ƫС��������Ӱ�족��
(3)����ͼ��ʾ������A��������_______________����ʵ������У���������¶ȼ��ϵ�����ˮ��ϴ�ɾ�ֱ�Ӳ���NaOH��Һ���¶ȣ����õġ�H -57.3KJ/mol�����������������������

(4)ʵ���и���80mL 0.50mol/L�����100mL 0.55 mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ�������ȡ�)��
�����к��� �����ȡ�������ȡ�) ��
(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �������ƫ����ƫС��������Ӱ�족��
(6)������ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������д��ϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ ��
��1�����·�ֹ������ʧ�� ��2��ƫС ��3�����β���������H��-57.3KJ/mol
��4������ȡ���� ��5��ƫС
��6��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l)����H=-57.3KJ/mol
����������1����ʵ����Ӧ�þ����ܵļ�����������ʧ�������ձ���������ĭ���ϵ������DZ��·�ֹ������ʧ��
(2)���ձ���������Ӳֽ�壬��������������ʧ���ⶨ���ƫ�͡�
��3�����������Ľṹ��֪��Ӧ�����ǻ��β��������������ܺ��������Ʒ�Ӧ�ų������������������������Һ����ʼ�¶ȸߣ���˵������շų�������ƫ�٣����H��-57.3KJ/mol��
��4���ı���ͼ����������Ӧ�зų���������仯������Ӧ���Dz���ģ���Ϊ�к�����ָ�������кͷ�Ӧ����1Ħ��H2Oʱ���ų��������������������ء�
��5����ˮ��������ʣ����ڵ���ƽ�⣬�������ȣ����Բ�õ��к��ȵ���ֵƫС��
��6�������к��ȵĸ����֪���Ȼ�ѧ����ʽΪ1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l)����H=-57.3KJ/mol��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺| �ζ����� | ������Һ�����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�
ʵ������50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ����е����ݣ�| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ����ͼװ�����к��ȵIJⶨʵ��
ijͬѧ����ͼװ�����к��ȵIJⶨʵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
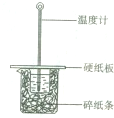 ijͬѧ��50mL 0.50mol/L��������50mL 0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ���ǣ�������
ijͬѧ��50mL 0.50mol/L��������50mL 0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������������к��ȣ�����˵����ȷ���ǣ�������| A��ʵ�������û��������ʧ | B���ձ���������ֽ���������ǹ̶�С�ձ� | C��ͼ��ʵ��װ��ȱ�ٻ��β�������� | D���������������Ϊ60mL�������������к��Ȳ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com