
��ĩ״����A���ɵ����ʵ�����MgO��Fe2O3��ɵĻ�����������ʵ�飺
��ȡ����A�������ȷ�Ӧ���������е���B���ɣ�
����ȡ20 g Aȫ������0.15 L 6.0 mol��L��1�����У�����ҺC��
�۽����еõ��ĵ���B����ҺC��Ӧ���ų�1.12 L(���)���壬ͬʱ������ҺD���������й�������B��
����KSCN��Һ����ʱ����ҺD����ɫ��
����գ�
(1)�����������ȷ�Ӧ��ʵ�������________�������еĵ���B��________��
(2)�����������ĸ���Ӧ�Ļ�ѧ����ʽ��_____________________________________
________________________________________________________________________��
(3)�����������ĸ���Ӧ�����ӷ���ʽ��_____________________________________
________________________________________________________________________��
(4)����ҺD���������Ϊ0.15 L�������Һ��c(Mg2��)Ϊ__________��c(Fe2��)Ϊ__________��
�𰸡�(1)������KClO3������þ���������ȼ��Fe
(2)Fe2O3��6HCl===2FeCl3��3H2O��
MgO��2HCl===MgCl2��H2O
(3)Fe��2H��===Fe2����H2����Fe��2Fe3��===3Fe2��
(4)0.67 mol��L��1��2.3 mol��L��1
���������⽫ʵ�������Ԫ�ػ�����֪ʶ��������һ�飬�������������������ǿ������IJ���������
(1)���ȷ�Ӧ��ָ����Al��ijЩ������������ķ�Ӧ����Ӧ�����зų������ȣ����÷�Ӧ��Ҫ�ϸߵ��¶Ȳ����������ڻ�����ϼ�����KClO3���岢����Mg������ȼMg����ų�������ʹKClO3����ֽ�ų�O2����һ�� �Ӿ�Mg��ȼ�գ����ڶ�ʱ����ʹ������¶�Ѹ�����ߣ�������Ӧ�������ķ�ӦΪFe2O3��2Al
�Ӿ�Mg��ȼ�գ����ڶ�ʱ����ʹ������¶�Ѹ�����ߣ�������Ӧ�������ķ�ӦΪFe2O3��2Al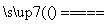 Al2O3��2Fe�����Բ����е���BΪFe��
Al2O3��2Fe�����Բ����е���BΪFe��
(2)Fe2O3��MgO���Ǽ���������ܺ��ᷴӦ�����κ�ˮ��Fe2O3��6HCl===2FeCl3��3H2O��MgO��2HCl===MgCl2��H2O��
(3)�������ֻ��Fe2O3�ܺ�Al�������ȷ�Ӧ������Fe���ʡ�C��Һ���з�Ӧ���ɵ�FeCl3������δ��Ӧ��HCl�����������ӷ�ӦΪFe��2H��===Fe2����H2����Fe��2Fe3��===3Fe2����
(4)���貽�����ȥ��20 g�����У�MgO�����ʵ���Ϊx����Fe2O3�����ʵ���ҲΪx�� ��40 g��mol��1x��160 g��mol��1x��20 g����ã�x��0.1 mol��
����MgO��MgCl2�Ĺ�ϵ������Һ��MgCl2��Ũ�� Ϊ
Ϊ
0��1 mol��0.15 L��0.67 mol��L��1��
�����˵����Һ��û��Fe3����Ҳ��������ΪFeCl2��MgCl2������Cl������Ĺ�ϵ����֪MgCl2��FeCl2���ܵ����ʵ�������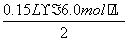 ��0.45 mol�����ԣ�FeCl2��Ũ��Ϊ
��0.45 mol�����ԣ�FeCl2��Ũ��Ϊ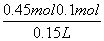 ��2.3 mol��L��1��
��2.3 mol��L��1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(һ) �����ֳ�̼�������Ǻ�������ߵĵ��ʣ���ҵ�����ö�����̼�Ͱ�����һ�������ºϳ����ء��䷴Ӧ��Ϊ����������
��һ����2NH3(l)��CO2(g) 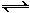 H2NCOONH4(���������) (l) ��H1= —330.0 kJ·mol��1
H2NCOONH4(���������) (l) ��H1= —330.0 kJ·mol��1
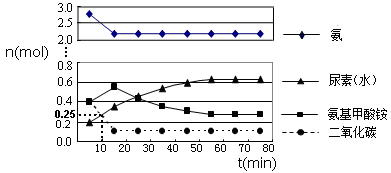
�ڶ�����H2NCOONH4(l) 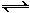 H2O(l)��H2NCONH2(l) ��H2= + 226.3 kJ·mol��1
H2O(l)��H2NCONH2(l) ��H2= + 226.3 kJ·mol��1
ijʵ��С��ģ�ҵ�Ϻϳ����ص���������һ���Ϊ0.5 m3 �ܱ�������Ͷ��4 mol����1mol������̼��ʵ���÷�Ӧ�и���ֵ����ʵ�����ʱ��ı仯����ͼ��ʾ��
����֪�ܷ�Ӧ�Ŀ���������һ����������ϳ������ܷ�Ӧ�Ŀ����ɵ� ����Ӧ������
�ڷ�Ӧ���е�10 minʱ���CO2�����ʵ�������ͼ��ʾ������CO2��ʾ�ĵ�һ����Ӧ������v(CO2)�� mol/(L·min)��
�۵���Ӧ��һ�������´ﵽƽ�⣬�����������������ٳ���һ��������He����CO(NH2)2(l)������_________(����ӡ�������С�����䡱)��
(��)�����Ʊ����ص�ԭ�ϣ�NH3��N2H4���ڹ�ũҵ���������պ���������й㷺Ӧ�á�
��������ˮ�õ���ˮ����25���£���amol/L�İ�ˮ��bmol/L��������3��2����Ȼ�Ϸ�Ӧ����Һ�����ԡ��ú�a��b�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ��Ϊ_________��
(��)�����Ǻϳɰ���ԭ�ϡ������ܡ�����δ�������������Դ��
(1)��25��,101KPa�����£�1 g������ȫȼ������Һ̬ˮʱ�ų�142.9kJ���������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
(2)����ͨ��������ˮú���ķ����Ƶá�����C(s)+ H2O(g)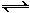 CO(g)+H2(g)����850��ʱƽ�ⳣ��K=1������1���ĺ㶨�ܱ����������ͬʱ����x mol C��6.0mol H2O��
CO(g)+H2(g)����850��ʱƽ�ⳣ��K=1������1���ĺ㶨�ܱ����������ͬʱ����x mol C��6.0mol H2O��
�ٵ����ȵ�850�淴Ӧ�ﵽƽ��ı�־��______________ ��
A�������ڵ�ѹǿ���� B������ˮ���������ʵ���������CO�����ʵ������
C����������ܶȲ��� D����λʱ����n��H-O�����ѵ�ͬʱ��n��H-H������
��xӦ����������� ��
(��)CO2�Ǻϳ����ص�ԭ�ϣ���ˮ�೧����ʱȴ�ŷų�������CO2����ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2���ŷţ������ԭ����ͼ��ʾ��
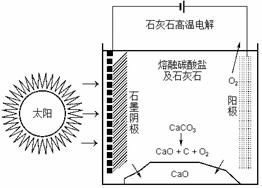
(1)�����������̵�����ת����ʽ�� ��
(2)������ⷴӦ���¶�С��900��ʱ����̼����ȷֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ �������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҩ�ﱴŵ����������ˮ����Ͷ�������������һ�������·�Ӧ�Ƶã�
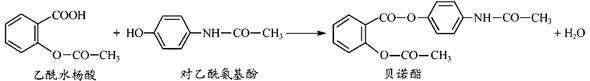
�й�������Ӧ��Ͳ����������ȷ����( )
A����̼�ĹǼܷ��࣬���������л�������ڷ�����
B������ˮ��������в���������̼ԭ��
C���ں˴Ź��������У������������ӷ�����4����
D����ŵ����������3�ֲ�ͬ���͵ĺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��EΪ����������Ԫ�أ����ǵ�ԭ����������������ش��������⡣
| A | Ԫ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| B | Ԫ��ԭ�ӵĺ���p��������s��������1 |
| C | ԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| D | Ԫ�ص������������������IJ�Ϊ3 |
| E | Ԫ���Ǹ����ڵ縺������Ԫ�� |
��1��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ����չ����ԭ�ӹ���� �Ρ�B��A���γɶ�����ʽ�Ļ��������BA5�������Ӿ��壬�����ʽΪ ��
��2��C�ĵ�����һ�ֳ����ķǽ����������о���ȼ�����ɺڡ������ֹ��壬д���÷�Ӧ�Ļ�ѧ����ʽ �����еİ�ɫ����������ͻ���ϣ������� ���塣
��3��BE3 �ڳ�������һ�ֵ���ɫ��Һ�壬�����侧�����֮����������� ������ˮ������ˮ�������һ�־���Ư���Ե����ʣ�д����Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ˵������B��E�зǽ����Խ�ǿ���� ����Ԫ�صķ��ţ���
��4��DO2����ͨ������Ba(BO3)2����Һ�����ɰ�ɫ��������ɫ���壬�йط�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
þ�������ʵĻ�ѧ����������Ϊ������Ҳ����ijЩ�ش�����ԣ���������������֤�����ߴ��ڽϴ�����Ե��� (����)
��CO2�������ᡡ��NaOH��Һ����ˮ
A���٢� B���ڢ� C���٢� D���ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������X������ˮ�������ռӦ������������ˮ�Ļ�����Y��������Y��Һ�����������������ɣ���X�� (����)
A��SiO2 B��Al2O3
C��MgO D��CuO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ���� (����)
A�����Ȼ�����Һ��ͨ�����������4NH3��Al3����4H2O===[Al(OH)4]����4NH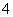
B������Ƭ��ĥ������NaOH��Һ�У�2Al��2OH����2H2O===2[Al(OH)4]����H2��
C����������Һ�м��������Ba(OH)2��Һ��Al3����2SO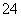 ��2Ba2����4OH��===2BaSO4����[Al(OH)4]��
��2Ba2����4OH��===2BaSO4����[Al(OH)4]��
D����Na[Al(OH)4]��Һ��ͨ������CO2��2[Al(OH)4]����CO2===2Al(OH)3����CO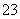 ��H2O
��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�п��ܺ���H����NH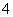 ��Mg2����Al3����Fe3����CO
��Mg2����Al3����Fe3����CO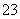 ��SO
��SO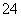 ��NO
��NO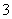 �еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ�
�еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ� ��֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���� (����)
��֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���� (����)
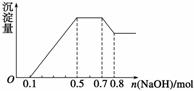
A����Һ�е�������ֻ��H����Mg2����Al3��
B����Һ��n(NH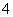 )��0.2 mol
)��0.2 mol
C����Һ��һ������CO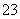 �����ܺ���SO
�����ܺ���SO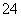 ��NO
��NO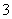
D��n(H��)��n(Al3��)��n(Mg2��)��1��1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��ȩ�ᷢ����Ӧ��RCHO+NaHSO3 RCH(OH)SO3Na���ȱ�������ȩ��ˮ�е��ܽ�Ⱥ�С�����ȱ��뱽��ȩ��Һ̬��������ķ����ǣ���NaHSO3��Һ����Һ�����ȱ����ټ�A���ʡ���Һ���ñ���ȩ����A���ʿ����ǣ� ��
RCH(OH)SO3Na���ȱ�������ȩ��ˮ�е��ܽ�Ⱥ�С�����ȱ��뱽��ȩ��Һ̬��������ķ����ǣ���NaHSO3��Һ����Һ�����ȱ����ټ�A���ʡ���Һ���ñ���ȩ����A���ʿ����ǣ� ��
A���Ҵ� B��NaOH C��NaCl D������KMnO4��Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com