
| �� | H��H | Br��Br | I��I | Cl��Cl | H��Cl | H��I | H��Br |
| ���� | 436 | 193 | 151 | 247 | 431 | 299 | 356 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2��SiO2 | B��CCl4��KCl | C��NaCl��HCl | D��CO2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ɱ� | B���Ȼ��� | C���������� | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��COS�������ӻ����� |
| B��COS�����У�����ԭ�Ӷ�����8���ӵ��ȶ��ṹ |
| C��COS�ĽṹʽΪO=C=S |
| D��COS���м��Թ��ۼ��ļ��Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢܢ� | B���ܢݢ� | C���ڢۢܢ� | D���ڢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
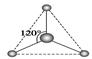 __________��
__________��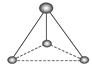 __________��
__________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij���ռ乹��Ϊƽ�������Σ�������ԭ��һ����sp2�ӻ� |
| B��ij���ռ乹��ΪV�Σ�������ԭ��һ���йµ��Ӷ� |
| C��ij���ռ乹��Ϊ�����Σ������һ���Ǽ��Է��� |
| D��ij���ռ乹��Ϊ�������壬�����һ����109��28�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com