
 �����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�
�����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã� ��
�� ����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ
����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ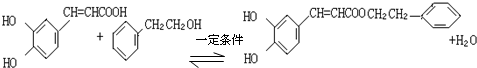 ��
�� ���� �ٺ����Ȼ�������̼�����Ʒ�Ӧ���ɶ�����̼���壻
�ڷ���ʽΪC17H16O4����������һ�������¿�ˮ�����ɿ������һ�ִ���˵��CPAE�����������ô�Ϊ���㴼�ҷ��ӽṹ�������ɷ���ʽ��֪�ô�Ϊ ���Դ˽��
���Դ˽��
��� �⣺�ٺ����Ȼ�������̼�����Ʒ�Ӧ���ɶ�����̼���壬��Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
�ڷ���ʽΪC17H16O4����������һ�������¿�ˮ�����ɿ������һ�ִ���˵��CPAE�����������ô�Ϊ���㴼�ҷ��ӽṹ�������ɷ���ʽ��֪�ô�Ϊ ����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ
����������÷��㴼��һ�������·�Ӧ����CPAE�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
���� ���⿼���л���Ľṹ�����ʣ���Ŀ�ѶȲ���ע���л���ĺ��еĹ����ŵ����ʣ���Ϊ������Ĺؼ�����Ϥ���ӡ�ϩ������������ʼ��ɽ��


 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | X��AlCl3��Mg��NO3��2��HNO3��Y��NaOH | |
| B�� | X��Na2CO3��NH4HCO3��Na2SO4��Y��Ba��OH��2 | |
| C�� | X��NH4NO3��Al��NO3��3��Fe��NO3��3��HCl��Y��NaOH | |
| D�� | X��NaAlO2����ˮ��NaOH��Y��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ͨ������Һ���� | B�� | �ڵ��ܿڴ���ȼ | ||
| C�� | һ����������H2�����ӳɷ�Ӧ | D�� | ������ͨ��������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ӧ���������淴Ӧ���ʼ�С | B�� | X��ת���ʱ�� | ||
| C�� | Y��ת���ʱ�� | D�� | ��ƽ��ʱX�����������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ⱥ��ʳ��ˮ�� ������Na++e-�TNa | |
| B�� | ���ͭʱ���������ҺΪCuSO4��Һ���� ������Cu-2e-�TCu2+ | |
| C�� | �������NaCl�� ������Na++e-�TNa | |
| D�� | ���NaOH��Һ�� ������4OH--4e-�T2H2O+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Ӧ���ɵ�����ˮ������Һ̬ˮ��ǰ�߷ų������� | |
| B�� | ��Ҫ���ȵķ�Ӧ˵���������ȷ�Ӧ | |
| C�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O����H=-57.3kJ/mol��������0.5molH2SO4��Ũ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ | |
| D�� | 1molS��ȫȼ�շ���297.3kJ�����Ȼ�ѧ����ʽ��S+O2�TSO2����H=-297.3 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.40mol/L | B�� | 0.30mol/L | C�� | 0.075mol/L | D�� | ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com