
 E��F��D��X��Y��ZΪǰ������Ԫ�أ���ԭ��������������E��������ۺ�����۵ľ���ֵ��ȣ�F�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Dԭ��δ�ɶԵ�������ͬ����Ԫ������࣬X��Dͬ���ڣ���һ�����ܱ�D�ͣ�Y��Fͬ���壬Z�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬����ش��������⣺
E��F��D��X��Y��ZΪǰ������Ԫ�أ���ԭ��������������E��������ۺ�����۵ľ���ֵ��ȣ�F�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Dԭ��δ�ɶԵ�������ͬ����Ԫ������࣬X��Dͬ���ڣ���һ�����ܱ�D�ͣ�Y��Fͬ���壬Z�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬����ش��������⣺���� E��F��D��X��Y��ZΪǰ������Ԫ�أ���ԭ��������������E������Ԫ����ԭ�Ӱ뾶��С�ģ���EΪHԪ�أ�F�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ���������Ų�Ϊ1s22s22p2����FΪCԪ�أ�Y��Fͬһ���壬���ԭ��������֪��YΪSi����Dδ�ɶԵ�������ͬ����Ԫ������࣬����Χ�����Ų�Ϊns2np3��ԭ������С��Si����DΪNԪ�أ�X��Dͬ���ڣ���һ�����ܱ�D�ĵͣ���XΪOԪ�أ�Z�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬��������Ϊ������Ԫ�أ�Ϊ��������Ԫ�أ���ʺ�������Ų�Ϊ1s22s22p63s23p63d104s1����ZΪCuԪ�أ��ݴ˽��
��� �⣺��1��Si�ĺ˵����Ϊ14�����������Ų�ʽΪ��1s22s22p63s23p2����̬Cuԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1����������ռ�ݵ�����ܲ�Ϊ���IJ㣬����ΪN��
�ʴ�Ϊ��1s22s22p63s23p2��N��
��2��Ԫ�صķǽ�����Խǿ���縺��Խ�ǽ�����O��N��C����縺����С�����˳��Ϊ���ʴ�Ϊ��O��N��C��
��3��H��C��N��O�γɵ��л���CO��NH2��2��������Cԭ���γ�3���Ҽ���û�йµ��Ӷԣ�Cԭ�Ӳ�ȡsp2�ӻ���Nԭ���γ�3���Ҽ�������1�Թµ��Ӷԣ�Nԭ�Ӳ�ȡsp3�ӻ���NO3-��Nԭ�Ӽ۲���ӶԸ�����3�Ҳ����µ��Ӷԣ�����Ϊƽ�������Σ�
�ʴ�Ϊ��sp2��sp3��ƽ�������Σ�
��4��C2H2�����к���̼̼��������ֱ���ͷ��ӣ���H2O2���Ӻ��зǼ��Թ��ۼ���
a��H2O2������ֻ���Ҽ�����b����
b��H2O2���ӷ��Ӽ�����������е����Ը���C2H2���ӣ�����ȷ��
c�����������Ǻ����Լ��ͷǼ��Լ��ļ��Է��ӣ��ʴ���
d�����۵����������ȣ����ǻ�Ϊ�ȵ����壬�ʴ���
��ѡ��b��
��5����Cu���ʵķ�ĩ���뵽NH3��Ũ��Һ�У���ͨ��O2����ַ�Ӧ����Һ������ɫ����Ӧ����[Cu��NH3��4]2+���÷�Ӧ�����ӷ���ʽΪ��2Cu+8NH3+O2+2H2O=2[Cu��NH3��4]2++4OH-��
�ʴ�Ϊ��2Cu+8NH3+O2+2H2O=2[Cu��NH3��4]2++4OH-��
��6��������Na+������ĿΪ8��O2-������ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Na+���ӡ�O2-������Ŀ֮��Ϊ2��1���ʸþ��廯ѧʽΪNa2O����ͼ��֪��ÿ��Na+������Χ��4��O2-���ӣ�Na+������λ��Ϊ4����һ����������Χ�����������������8����8�������ӹ��ɵļ�����Ϊ�������壬����ԭ���������AΪ��0��0��0����BΪ��$\frac{1}{2}$��0��$\frac{1}{2}$����CΪ��$\frac{1}{2}$��$\frac{1}{2}$��0������Dԭ�Ӽ�Naԭ�ӵ��������Ϊ��$\frac{1}{4}$��$\frac{1}{4}$��$\frac{1}{4}$��������O2-�����붥��O2-���Ӿ����������������Ϊ$\frac{4��62}{{N}_{A}}$g���þ������ܶ�Ϊ��g•cm-3�����������Ϊ$\frac{4��62}{{N}_{A}}$g�¦�g•cm-3=$\frac{248}{��{N}_{A}}$cm3�������ⳤΪ$\root{3}{\frac{248}{��{N}_{A}}}$cm�������������W���Ӽ����Ϊ$\root{3}{\frac{248}{��{N}_{A}}}$��$\frac{\sqrt{2}}{2}$cm=$\root{3}{\frac{248}{��{N}_{A}}}$��$\frac{\sqrt{2}}{2}$��10-7nm��
�ʴ�Ϊ��Na2O��4����$\frac{1}{4}$��$\frac{1}{4}$��$\frac{1}{4}$����$\root{3}{\frac{248}{��{N}_{A}}}$��$\frac{\sqrt{2}}{2}$��10-7��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����ӻ���������ӹ��͡��������������ʡ����������ṹ�����ȣ���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H6O2 | B�� | C2H4O2 | C�� | C3H8O | D�� | C2H6O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3L | B�� | 2L | C�� | 1L | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
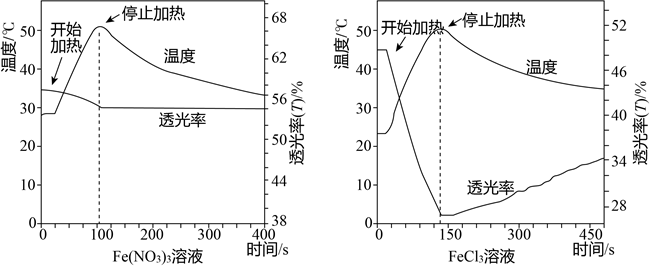
| ��� | ʵ�� | ���������� |
| a | ��Fe��NO3��3��Һ���ػ�ɫ���м������HNO3 | i����Һ��ɫ�dz�dz ii�����Ⱥͽ��¹��������������Ա仯 |
| b | ��FeCl3��Һ����ɫ���м������HNO3 | i����Һ��Ϊ��ɫ ii�����������½��������������� |
| ��� | ʵ�� | ���������� |
| c | ��Fe��NO3��3��Һ�м������HNO3���ټ��뼸��NaCl��Һ�� | �����Һ�������¶ȸı�ı仯���������������Ũ�ȣ���ɫ����Ũ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

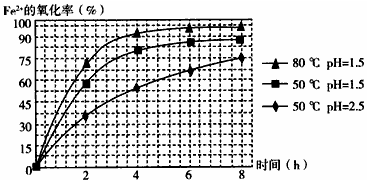
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | Fe3+��I-��Cl-��������һ������ | |
| B�� | CO32-һ�������ڣ�����ȷ��Na+��Cl-�Ƿ���� | |
| C�� | Fe3+��Fe2+������һ�� | |
| D�� | ����Һ��c��Cl-������Ϊ0.2mol•L?1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4+H2O��CO+3H2 | B�� | CH4+F2��CH3F+HF | C�� | 2CH4��C+2H2 | D�� | 2CH4��C2H2+3H2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com