
ʹ�����ֻ�ʵ���ң��ɵ��ԡ����ݲɼ�����ר�ô����������ֻ�̽ͷ�Լ�ƽ��ʹ�õĻ�ѧ�������Լ�����ɣ���ù���0.1mol/L��CH3COONa��Һ�ļ������ݣ�����ʱ0.1mol/L��CH3COONa��Һ��pHΪ8.75�������½����ˮϡ�͵�0.01mol/L, ��pHΪ8.27������0.1mol/L��CH3COONa��Һ���ȣ����ȹ�����pH�ı仯����ͼ��
������˵���������ǣ�
A������ʵ�����ݶ�����˵��CH3COONa��Һ�д���ˮ��ƽ��
B��CH3COONaˮ��ƽ�ⳣ��
C�������½�0.1mol/L CH3COONa��Һ��ˮϡ�͵�0.01mol/L��pH��8.75�仯��8.27������˵��0.1mol/LCH3COONa��Һˮ��̶ȸ���
D������0.1mol/L��CH3COONa��Һ��pH�ı仯��˵���¶����ߣ�K����
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ȿ����������������ϩ���ֿɳ�ȥ�����л�����ϩ����ѷ����ǣ� ��
A��ͨ�����Ը��������Һ�� B��ͨ��������ˮ��
C��һ��������ͨ��H2 D����ȼ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������У��ܷ�����������ԭ���ӳɡ���ȥ���ַ�Ӧ���� �� ��
A��CH3��CH��CH��CHO B��HOCH2��CH2��CH��CH��CHO
C��CH3�� ��
�� ��CH3 D��HOCH2��
��CH3 D��HOCH2�� ��CH2��CHO
��CH2��CHO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ�ý���ʵ�飬��Һ��A��μ��뵽����B�У� ���������д�����ǣ� ��
A��ʵ��������C����ֹ����������
B. ��AΪ���ᣬBΪ���ǣ���״����D��ʢC6H5ONa
��Һ����D����Һ�����
C. ��AΪŨ��ˮ��BΪ��ʯ�ң�D��ʢAgNO3��Һ��
��D��������
D. ��AΪʳ��ˮ��BΪ��ʯ��D��ʢKMnO4������Һ����D
����Һ�Ϻ�ɫ��ɫ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����Һ�еμ���һ����Һ����Һ����ɫ�����������仯����
A���Ȼ�������Һ�м���˫��ˮ B����������Һ�еμ����������Һ
C������ͭ��Һ�еμ����ᱵ��Һ D���������������Һ�еμ�����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.�����й�˵������ȷ����___ _____��
_____��
A����ͬ���͵����Ӿ��壬������Խ���γɵľ���Խ�ȶ�
B��NH3��H3O+�ǵȵ����壬��˽ṹ����������
C�����ǻ�����ȩ�е���ڶ��ǻ�����ȩ��ԭ����ǰ�ߴ��ڷ�����������ߴ���
���Ӽ����
D��H3O����HF2����[Ag(NH3)2]���о�������λ��
��.̼���仯��������Ȼ���й㷺���ڡ�
(1)��̬̼ԭ�ӵļ۵����Ų�ͼ�ɱ�ʾΪ ������������������ͬ
δ�ɶԵ������Ĺ��ɽ����� ����Ԫ�ط��ţ�
(2)��һ�����ܣ�C��N��O��F����Ԫ���ɴ�С˳��___ _ ��
ԭ���� ��
HCN��NF3���ӹ��ͷֱ��� ��
(3)��������ˮ���ӵĿռ����з�ʽ����ʯ�������ơ�ÿ��������ƽ��ռ��________��ˮ���ӣ�����������ʯ�������з�ʽ��ͬ��ԭ����__________________________��
(4)C60�ľ����У�����Ϊ���������ѻ�����֪������C60�������ļ����̾���Ϊ
d cm���ɼ���C60������ܶ�Ϊ________g/cm3��
(5)��д��һ����Ӧ����ʽ�Ա������Ӧǰ̼ԭ�ӵ��ӻ���ʽΪsp2����Ӧ���Ϊsp3��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д��ȷ����
A��������SO2����ͨ��NaClO��Һ�� SO2 +2ClO��+H2O = SO32��+2HClO
B����KHSO4��Һ�м���Ba(OH)2��Һ��������Һ��pH=7 Ba2+ +OH��+ H++ SO42�� = BaSO4��+ 2H2O
C����Ca(H2PO4)2��Һ�е��������NaOH��Һ 3Ca2+ + 6H2PO4��+12OH��= Ca3(PO4)2��+ 4PO43��+12H2O
D��112mL�������Cl2ͨ��10mL1mol/L��FeBr2��Һ�� 2Fe2+ + 4Br��+3Cl2 = 2Fe3+ + 6Cl��+2Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʵ����������ͺ�����ͬ��ͬѹ�¾�����ȵ�
A��ԭ���� B���ܶ� C�������� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ�����ʽṹ������]ʯīϩ[��ͼ(a)��ʾ]��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ[��ͼ(b)��ʾ]��
 ��
��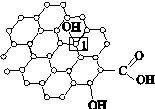
(a)ʯīϩ�ṹ����������(b)����ʯīϩ�ṹ
(1)ͼ(a)�У�1��C������C�γɦҼ��ĸ���Ϊ________��
(2)ͼ(b)�У�1��C���ӻ���ʽ��________����C������C�γɵļ���________(�>����<������)ͼ(a)��1��C������C�γɵļ��ǡ�
(3)����ͼ(b)��ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����________(��Ԫ�ط���)��
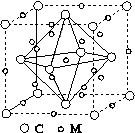
(4)ʯīϩ��ת��Ϊ����ϩ(C60)��ij����M��C60���Ʊ�һ�ֵ��³������ϣ�������ͼ��ʾ��Mԭ��λ�ھ������������ڲ����þ�����Mԭ�ӵĸ���Ϊ________���ò��ϵĻ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com