
ÓÉÓŚĪĀŹŅŠ§Ó¦ŗĶ׏Ō“¶ĢȱµČĪŹĢā£¬ČēŗĪ½µµĶ“óĘųÖŠµÄCO2ŗ¬Įæ²¢¼ÓŅŌæŖ·¢ĄūÓĆ£¬ŅżĘšĮĖø÷¹śµÄĘÕ±éÖŲŹÓ”£ÄæĒ°¹¤ŅµÉĻÓŠŅ»ÖÖ·½·ØŹĒÓĆCO2Éś²śČ¼ĮĻ¼×“¼”£Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)+3H2(g) CH3OH(g)+H2O(g)£¬ČēĶ¼±ķŹ¾øĆ·“Ó¦½ųŠŠ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ·mol£1)µÄ±ä»Æ”£
CH3OH(g)+H2O(g)£¬ČēĶ¼±ķŹ¾øĆ·“Ó¦½ųŠŠ¹ż³ĢÖŠÄÜĮæ(µ„Ī»ĪŖkJ·mol£1)µÄ±ä»Æ”£
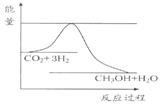 £Ø1£©¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬Ęä”÷H 0”£(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)£¬ ĒŅŌŚ £ØĢī”°½Ļøß”±»ņ”°½ĻµĶ”±£©ĪĀ¶ČĻĀÓŠĄūÓŚøĆ·“Ó¦×Ō·¢½ųŠŠ”£
£Ø1£©¹ŲÓŚøĆ·“Ó¦µÄĻĀĮŠĖµ·ØÖŠ£¬Ęä”÷H 0”£(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)£¬ ĒŅŌŚ £ØĢī”°½Ļøß”±»ņ”°½ĻµĶ”±£©ĪĀ¶ČĻĀÓŠĄūÓŚøĆ·“Ó¦×Ō·¢½ųŠŠ”£
£Ø2£©øĆ·“Ó¦Ę½ŗā³£ŹżKµÄ±ķ“ļŹ½ĪŖ ”£
£Ø3£©ĪĀ¶Č½µµĶ£¬Ę½ŗā³£ŹżK (Ģī”°Ōö“ó”±”¢”°²»±ä”±»ņ”°¼õŠ””±)”£
£Ø4£©ČōĪŖĮ½øöČŻ»żĻąĶ¬µÄĆܱÕČŻĘ÷,ĻÖĻņ¼×ČŻĘ÷ÖŠ³äČė1 mol CO2(g)ŗĶ3 molH2(g)£¬ŅŅČŻĘ÷ÖŠ³äČė1mol CH3OH(g)ŗĶ1 mol H2O(g)£¬ŌŚĻąĶ¬µÄĪĀ¶ČĻĀ½ųŠŠ·“Ó¦,“ļµ½Ę½ŗāŹ±,¼×ČŻĘ÷ÄŚn(CH3OH)”””” (Ģī”°“óÓŚ”±”°Š”ÓŚ”±»ņ”°µČÓŚ”±)ŅŅČŻĘ÷ÄŚn(CH3OH)”£
£Ø5£©ŅŃÖŖ£ŗCO(g)+2H2(g) = CH3OH (g) ”÷H= -116 kJ•mol-1£»CO(g)+1/2O2(g)=CO2(g)
”÷H=-283 kJ•mol-1 £»H2 (g)+1/2O2(g)=H2O(g) ”÷H=-242 kJ•mol-1 ,Š“³öCH3OHČ¼ÉÕÉś³ÉCO2ŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³ĢŹ½______________________________________”£
£Ø6£©ŅŌ¼×“¼ŗĶŃõĘųĪŖČ¼ĮĻ£¬ĒāŃõ»Æ¼ŲČÜŅŗĪŖµē½āÖŹČÜŅŗ¹¹³Éµē³Ų”£
¢Łøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
¢ŚČōŅŌŹÆÄ«ĪŖµē¼«£¬µē½āĮņĖįĶČÜŅŗ£¬Š“³öµē½āµÄ×Ü·“Ó¦·½³ĢŹ½ ”£ČōŅŌ“ĖČ¼ĮĻµē³Ųµē½ā200 mL 0.8mol/LµÄĮņĖįĶČÜŅŗ£¬µ±ĻūŗÄ1.6¼×“¼Ź±£¬ŌŚŅõ¼«»įĪö³ö gĶ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
“óĮæČ¼ÉÕ»ÆŹÆČ¼ĮĻŅŌ¼°¹¤³§Ī²ĘųµÄÅÅ·ÅŹĒæÕĘųĪŪČ¾µÄÖ÷ŅŖŌŅņ”£Ēė»Ų“š£ŗ
£Ø1£©ĻĀĮŠĘųĢåµÄÅÅ·ÅÄÜŠĪ³ÉĖįÓźµÄŹĒ £ØĢīŠņŗÅ£©”£
¢Ł CO2 ¢Ś SO2 ¢Ū NO2
£Ø2£©ŌŚČ¼ĆŗÖŠ¼ÓČėŅ»ÖÖĪļÖŹ£¬æɼõÉŁSO2µÄÅÅ·Å”£Ķس£¼ÓČėµÄĪļÖŹŹĒ £ØĢīŠņŗÅ£©”£
¢Ł ÉÕ¼ī ¢Ś ŹÆ»ŅŹÆ ¢Ū ŹÆøą£ØCaSO4£©
£Ø3£©½«Ćŗ½ųŠŠĘų»Æ£¬ŌŁČ¼ÉÕŹ±æÉŅŌ“ó“ó¼õÉŁSO2ŗĶŃĢ³¾¶Ō“óĘųµÄĪŪČ¾£¬ĒŅČ¼ÉÕŠ§ĀŹøß”£ĆŗĘų»ÆµÄ¹ż³ĢČēĻĀ£ŗ
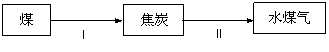 |
¹ż³ĢII·“Ó¦µÄĄąŠĶŹĒ £ØĢīŠņŗÅ£©”£
¢Ł ÖĆ»»·“Ó¦ ¢Ś ø“·Ö½ā·“Ó¦ ¢Ū Ńõ»Æ»¹Ō·“Ó¦
£Ø4£©ĮņĖį¹¤ŅµĪ²ĘųÖŠµÄSO2£¬³£ÓĆ×ćĮæµÄŹÆ»ŅČéĪüŹÕ”£Čō“¦Ąķŗ¬SO2 0.112%£ØĢå»ż·ÖŹż£©µÄĪ²Ęų1”Į106 m3£Ø±ź×¼×“æö£©£¬ĄķĀŪÉĻÄܵƵ½CaSO3 kg”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĶ¬Ń§ĪŖ¼ģŃéČÜŅŗÖŠŹĒ·ńŗ¬ÓŠ³£¼ūµÄĖÄÖÖĪŽ»śĄė×Ó£¬½ųŠŠĮĖĻĀĶ¼ĖłŹ¾µÄŹµŃé²Ł×÷”£ĘäÖŠ¼ģŃé¹ż³ĢÖŠ²śÉśµÄĘųĢåÄÜŹ¹ŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£ÓÉøĆŹµŃéÄܵƵ½µÄÕżČ·½įĀŪŹĒ(””””)
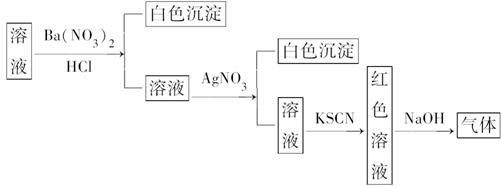
A£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO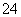
B£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNH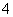 Ąė×Ó
Ąė×Ó
C£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCl£Ąė×Ó
D£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe3£«Ąė×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
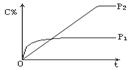
ŌŚĪĀ¶ČĻąĶ¬£¬Ń¹Ēæ·Ö±šĪŖp1”¢p2Ģõ¼žĻĀ£¬A(g)£«2B(g)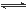 nC(g)µÄ·“Ó¦ĢåĻµÖŠ,CµÄĢå»ż·ÖŹż(C£„)Ėꏱ¼ä(t)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®
nC(g)µÄ·“Ó¦ĢåĻµÖŠ,CµÄĢå»ż·ÖŹż(C£„)Ėꏱ¼ä(t)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®
ĻĀĮŠ½įĀŪÕżČ·µÄŹĒ
A£®p1 > p2 n < 3 B£®p1 < p2 n > 3
C£®p1 < p2 n £½ 3 D£®p1 > p2 n > 3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚµē½āÖŹČÜŅŗµÄŠšŹöÕżČ·µÄŹĒ
A£®³£ĪĀĻĀ£¬pH£½7µÄNH4ClÓė°±Ė®µÄ»ģŗĻČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”Ė³ŠņĪŖ£ŗ
c (Cl£) > c (NH4+) > c (H£«)> c (OH£)
B£®½«pH£½4µÄ“×ĖįČÜŅŗĻ”ŹĶŗó£¬ČÜŅŗÖŠĖłÓŠĄė×ÓµÄÅØ¶Č¾ł½µµĶ
C£®ÖŠŗĶpHÓėĢå»ż¾łĻąĶ¬µÄŃĪĖįŗĶ“×ĖįČÜŅŗ£¬ĻūŗÄNaOHµÄĪļÖŹµÄĮæĻąĶ¬
D£®³£ĪĀĻĀ£¬Ķ¬ÅØ¶ČµÄNa2SÓėNaHSČÜŅŗĻą±Č£¬Na2SČÜŅŗµÄpH“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ( )
A£®±ź×¼×“æöĻĀ£¬22.4LCCl4ŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA
B£®³£ĪĀ³£Ń¹ĻĀ£¬17gNH3 Ėłŗ¬µÄŌ×ÓŹżÄæĪŖ4NA
C£®1 mol Na2O2ÓėH2OĶźČ«·“Ó¦£¬×ŖŅĘ2NAøöµē×Ó
D£®0.1mol/LNa2CO3ČÜŅŗÖŠŗ¬ÓŠµÄNa+ŹżÄæĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ (””””)
A£®ÅØĻõĖįÖŠ¼ÓČė¹żĮæĢś·Ū²¢¼ÓČČ£ŗFe£«3NO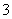 £«6H£«=Fe3£«£«3NO2”ü£«3H2O
£«6H£«=Fe3£«£«3NO2”ü£«3H2O
B£®Ca(HCO3)2ČÜŅŗÓė¹żĮæNaOHČÜŅŗ·“Ó¦ HCO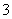 £«OH££«Ca2£«===CaCO3”ż£«H2O
£«OH££«Ca2£«===CaCO3”ż£«H2O
C£®ĒāŃõ»ÆĢśČÜÓŚĒāµāĖįČÜŅŗ£ŗFe(OH)3£«3H£«===Fe3£«£«3H2O
D£®µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»Æ±µČÜŅŗÓėĢ¼ĖįĒāļ§ČÜŅŗ»ģŗĻ£ŗ
Ba2£«£«2OH££«NH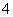 £«HCO
£«HCO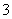 ===BaCO3”ż£«NH3”¤H2O£«H2O
===BaCO3”ż£«NH3”¤H2O£«H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®Ķ¬Ö÷×å·Ē½šŹōŌŖĖŲµÄ¼ņµ„ŅõĄė×ӵĻ¹ŌŠŌŌ½Ē棬ĘäŌŖĖŲ·Ē½šŹōŠŌŌ½Ēæ
B£®¢ńA×åÓė¢÷A×åŌŖĖŲ¼äæÉŠĪ³É¹²¼Ū»ÆŗĻĪļ»ņĄė×Ó»ÆŗĻĪļ
C£®ŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżµČÓŚŌŖĖŲµÄ×īøß»ÆŗĻ¼Ū
D£®Č«²æÓÉ·Ē½šŹōŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļÖŠÖ»ŗ¬¹²¼Ū¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ęĻ¶š¶žŗÅŌĀĒņĢ½²āĘ÷»ńµĆĮĖ7 m·Ö±ęĀŹČ«ŌĀĒņÓ°ĻńĶ¼£¬øĆŌĀĒņĢ½²āĘ÷Ź¹ÓĆīŠŌŖĖŲµÄĶ¬Ī»ĖŲµē³ŲĄ“Ģį¹©ĪČ¶Ø”¢³Ö¾ĆµÄÄÜŌ“”£ĻĀĮŠ¹ŲÓŚ PuµÄĖµ·ØÕżČ·µÄŹĒ(””””)
PuµÄĖµ·ØÕżČ·µÄŹĒ(””””)
A. PuŗĶ
PuŗĶ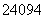 PuµÄÖŹ×ÓŹżÖ®²īĪŖ2
PuµÄÖŹ×ÓŹżÖ®²īĪŖ2
B. PuŗĶ
PuŗĶ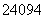 PuŹĒĮ½ÖÖ²»Ķ¬µÄŗĖĖŲ
PuŹĒĮ½ÖÖ²»Ķ¬µÄŗĖĖŲ
C. PuµÄÖŠ×ÓŹżÓėÖŹ×ÓŹżÖ®²īĪŖ144
PuµÄÖŠ×ÓŹżÓėÖŹ×ÓŹżÖ®²īĪŖ144
D. PuŗĶ
PuŗĶ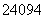 Pu»„ĪŖĶ¬ĖŲ
Pu»„ĪŖĶ¬ĖŲ ŅģŠĪĢå
ŅģŠĪĢå
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com