
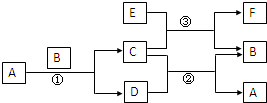
 ÓŅĶ¼ÖŠĖłÉę¼°µÄA”¢B”¢C”¢D”¢E”¢FŗĶG¶¼ŹĒ֊ѧ»Æѧ½Ģ²ÄÖŠ³£¼ūµÄĪļÖŹ£®EĪŖĀĮ£»B”¢DĪŖµ„ÖŹ£¬ĘäÓąĪŖ»ÆŗĻĪļ£»·“Ó¦¢Ł¢Ś¢Ū¾łŌŚøßĪĀĻĀ½ųŠŠĒŅ¾łĪŖÖĆ»»·“Ó¦£»AŌŚ³£ĪĀĻĀĪŖŅŗĢ¬£¬CÓŠ“ÅŠŌ£¬E”¢F¼ČÄÜČÜÓŚNaOHÓÖÄÜČÜÓŚHCl£®
ÓŅĶ¼ÖŠĖłÉę¼°µÄA”¢B”¢C”¢D”¢E”¢FŗĶG¶¼ŹĒ֊ѧ»Æѧ½Ģ²ÄÖŠ³£¼ūµÄĪļÖŹ£®EĪŖĀĮ£»B”¢DĪŖµ„ÖŹ£¬ĘäÓąĪŖ»ÆŗĻĪļ£»·“Ó¦¢Ł¢Ś¢Ū¾łŌŚøßĪĀĻĀ½ųŠŠĒŅ¾łĪŖÖĆ»»·“Ó¦£»AŌŚ³£ĪĀĻĀĪŖŅŗĢ¬£¬CÓŠ“ÅŠŌ£¬E”¢F¼ČÄÜČÜÓŚNaOHÓÖÄÜČÜÓŚHCl£® Fe3O4+4H2£¬¹Ź“š°øĪŖ£ŗ3Fe+4H2O
Fe3O4+4H2£¬¹Ź“š°øĪŖ£ŗ3Fe+4H2O Fe3O4+4H2£»
Fe3O4+4H2£» 4Al2O3+9Fe£¬¹Ź“š°øĪŖ£ŗ3Fe3O4+8Al
4Al2O3+9Fe£¬¹Ź“š°øĪŖ£ŗ3Fe3O4+8Al 4Al2O3+9Fe£®
4Al2O3+9Fe£®


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
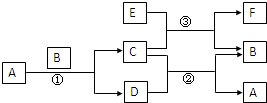 ÓŅĶ¼ÖŠĖłÉę¼°µÄA”¢B”¢C”¢D”¢E”¢FŗĶG¶¼ŹĒ֊ѧ»Æѧ½Ģ²ÄÖŠ³£¼ūµÄĪļÖŹ£®EĪŖĀĮ£»B”¢DĪŖµ„ÖŹ£¬ĘäÓąĪŖ»ÆŗĻĪļ£»·“Ó¦¢Ł¢Ś¢Ū¾łŌŚøßĪĀĻĀ½ųŠŠĒŅ¾łĪŖÖĆ»»·“Ó¦£»AŌŚ³£ĪĀĻĀĪŖŅŗĢ¬£¬CÓŠ“ÅŠŌ£¬E”¢F¼ČÄÜČÜÓŚNaOHÓÖÄÜČÜÓŚHCl£®
ÓŅĶ¼ÖŠĖłÉę¼°µÄA”¢B”¢C”¢D”¢E”¢FŗĶG¶¼ŹĒ֊ѧ»Æѧ½Ģ²ÄÖŠ³£¼ūµÄĪļÖŹ£®EĪŖĀĮ£»B”¢DĪŖµ„ÖŹ£¬ĘäÓąĪŖ»ÆŗĻĪļ£»·“Ó¦¢Ł¢Ś¢Ū¾łŌŚøßĪĀĻĀ½ųŠŠĒŅ¾łĪŖÖĆ»»·“Ó¦£»AŌŚ³£ĪĀĻĀĪŖŅŗĢ¬£¬CÓŠ“ÅŠŌ£¬E”¢F¼ČÄÜČÜÓŚNaOHÓÖÄÜČÜÓŚHCl£®
| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŌĘÄĻŹ”Ą„Ć÷ČżÖŠ”¢µį³Ų֊ѧøßŅ»£ØÉĻ£©ĘŚÄ©»ÆѧŹŌ¾ķ£Ø¶ž£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
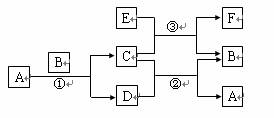
ÓŅĶ¼ÖŠĖłÉę¼°µÄA”¢B”¢C”¢D”¢E”¢FŗĶGµČ¶¼ŹĒ֊ѧ»Æѧ½Ģ²ÄÖŠ³£¼ūµÄĪļÖŹ”£·“Ó¦¢Ł¢ŚŹĒÖĆ»»·“Ó¦£¬·“Ó¦¢Ł¢Ś¢Ū¾łŌŚøßĪĀĻĀ½ųŠŠ”£AŌŚ³£ĪĀĻĀĪŖŅŗĢ¬£¬CÓŠ“ÅŠŌ£¬F¼ČÄÜČÜÓŚNaOHÓÖÄÜČÜÓŚHCl”£
¢ÅAµÄµē×ÓŹ½______________________£»
¢ĘŠ“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½____________________________________________£»
¢ĒŠ“³ö·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½£ŗ____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com