
����Ŀ�����⻯��(NaBH4����Ϊ+3��)Ϊ��ɫ��ĩ���ڸ���������ȶ����ڳ�ʪ�����зֽ⣬�dz��õĻ�ԭ����ƫ�����ƣ�NaBO2��������ˮ���������Ҵ�����ˮ�⡣Ŀǰ�ж��ֹ��տ��Ʊ�NaBH4��
��1��������һ����B2O3����Al2O3��SiO2��Fe2O3�����ʣ���ȡNaBH4���������£�

�١��ܽ���ʱ��B2O3��NaOH��Ӧ������NaBO2����Ӧ���ӷ���ʽΪ____��
�ڡ����������������CaO����������CaCl2��ԭ���У��ܽ��衢���Գ�����ȥ�������������������ӣ�___��
�ۡ�����2���ǽ���Һ�������ᾧ��ϴ�ӣ�����ϴ��ѡ�õ��Լ������_____������ĸ����
a. ��ˮ���� b. �Ҵ��� c. ��ˮ���� d. NaOH��Һ
������Ӧ1����MgH2��NaBO2��ϵõ�NaBH4��MgO���仯ѧ����ʽΪ________��
��2���ҹ�����ƽ������ʴ�缫���ϣ��������ӽ���ĤΪ����Ĥ�����ƫ�����Ƶļ���Һ��Ҳ���Ը�Ч�Ʊ�NaBH4���ù�����������Ϊ________�������缫����ʽΪ_____��
���𰸡�B2O3+2OH- =2BO2-+H2O ʹ��Һ�ʼ��ԣ�����NaBO2 ��ˮ�� b 2MgH2+NaBO2 =NaBH4+2MgO O2 BO2-+8e-+6H2O=BH4-+8OH-
��������
��1���������м����������Ʒ�����Ӧ��B2O3+2NaOH =2NaBO2+H2O��Al2O3+2NaOH=2NaAlO2+H2O��SiO2+2NaOH=Na2SiO3+H2O�����ˡ���������Fe2O3����Һ�м���CaO������Na2O��3CaO��Al2O3��nSiO2����,���˳���������ͬʱ���Ի�������NaBO2��ˮ�⣬��Һ�������ᾧ��ϴ�ӣ��õ�NaBO2��NaBO2��MgH2��Ӧ����NaBH4��
NaBO2��MgH2����NaBH4������þ������
��2�����ݵ�������������ʧ���ӣ�������ԭ�õ��ӣ��ж����������д�缫��Ӧʽ��
��1����B2O3��NaOH��Ӧ������NaBO2��ˮ����Ӧ���ӷ���ʽΪB2O3+2OH- =2BO2-+H2O��
�𰸣�B2O3+2OH- =2BO2-+H2O
�������������������CaO��CaO+H2O=Ca(OH)2,��Һ�ʼ��ԣ���������NaBO2 ��ˮ�⣻
�𰸣�ʹ��Һ�ʼ��ԣ�����NaBO2 ��ˮ��
��������2���ǽ���Һ�������ᾧ��ϴ�ӣ��õ�NaBO2����Ϊƫ�����ƣ�NaBO2��������ˮ���������Ҵ�����ˮ�⣬����ϴ��ѡ�õ��Լ�������Ҵ�����ѡb��
�𰸣�b
������Ӧ1����MgH2��NaBO2��ϵõ�NaBH4��MgO�����������غ�ɵã���ѧ����ʽΪ2MgH2+NaBO2 =NaBH4+2MgO��
�𰸣�2MgH2+NaBO2 =NaBH4+2MgO
��2�����ƫ�����Ƶļ���Һ��������8OH--8e-=4H2O+2O2��������BO2-+8e-+6H2O=BH4-+8OH-��
�𰸣�BO2-+8e-+6H2O=BH4-+8OH-



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z�ǵ���A����A������ַǽ���Ԫ�أ�������Ԫ�����ڱ��е�λ����ͼ��ʾ���Իش��������⡣
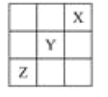
��1��XԪ�ص��ʵĻ�ѧʽ��________��
��2��YԪ�ص�ԭ�ӽṹʾ��ͼ��____________��Y��Na���γɻ�����ĵ���ʽΪ________________________��
��3��ZԪ�ص�������________����Ԫ��ԭ�ӵ�ʧ���ӵĽǶȿ���ZԪ�ؾ���________�ԣ�����ZԪ����Ԫ�����ڱ�������λ�ÿ����������������ʵ�ԭ����_________________________����۵����Ų�ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ba(OH)2 ��һ��ǿ������ڲⶨ��Ȼ���� CO2 �ĺ�����
��1��CO2 ���ܶȱȿ���______��������������С����
��2������ȡ5.25g ����[���� Ba(OH)2xH2O ������]��� 100 mL ��Һ��������Һ���õ�����������ƽ��ҩ�ס���Ͳ���ձ�����������______����ͷ�ιܡ��� 15.00 mL 0.2 mol/L���������� Ba(OH)2 ��Һ��Ӧ�����ĸ� Ba(OH)2��Һ 10.00 mL�����ʲ����ᷴӦ��
����ȡ5.25 g ����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�ʣ���������Ϊ3.09 g�� �� Ba(OH)2xH2O�е� x=______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����������A�Ļ�ѧʽΪC6H12O2����֪��

��֪D����Na2CO3��Ӧ��C��E�����ܷ���������Ӧ����A�Ľṹ�����У�������
A. 1�� B. 2�� C. 3�� D. 4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ˮ�к�5.00��10-3mol��L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
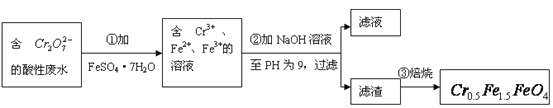
��1����������Ӧ�����ӷ���ʽ��_________________________________________________��
��2������������pH��ֽ�ⶨ��ҺpH�IJ����ǣ�
______________________________________________________________________________��
��3�����������˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����______________________��
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ����__________g FeSO4��7H2O��
����������Ҫ����__________g FeSO4��7H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼�����ﺬ���Ե���Ϊ��Ҫ��
�������������о�
��1��һ�������£���2molNO��2molO2���ں����ܱ������з�����Ӧ2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����____________��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����____________��
a����ϵѹǿ���ֲ��� b����������ܶȱ��ֲ���
c��NO��O2�����ʵ���֮�ȱ��ֲ��� d��ÿ����2molNOͬʱ����2 molNO2
��2��������ȼ������ʱ������N2��O2�ķ�Ӧ��N2+ O2![]() 2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2��g��+ O2��g��
2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2��g��+ O2��g��![]() 2NO��g���ġ�H____0(����������������)��
2NO��g���ġ�H____0(����������������)��

��3��NOx������β���е���Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ��
�������仯��ͼ��ʾ��
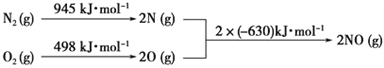
д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________��
������������͵����������dz��õĻ���ԭ������Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��1�����������У�SO2����������SO3��2SO2(s)+O2��g��![]() 2SO3��g����ij�¶��£�SO2��ƽ��ת����(��)����ϵ��ѹǿ(P)�Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3��g����ij�¶��£�SO2��ƽ��ת����(��)����ϵ��ѹǿ(P)�Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
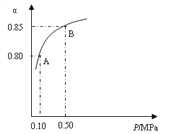
�ٽ�2.0 molSO2��1.0molO2����10 L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa���÷�Ӧ��ƽ�ⳣ������__________��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��_______K(B)(���������������=��)��
��2����CH4����ԭNOx�������������������Ⱦ��������
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ��mol-1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ��mol-1
���ñ�״����4.48 L CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ__________(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ___________kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ����
A. pH=3��ǿ����Һ1mL����ˮϡ����100mL����ҺpH����2����λ
B. 1L 0.50mol��L-1NH4Cl ��Һ��2L 0.25mol��L-1NH4Cl ��Һ��NH4+���ʵ������ߴ�
C. pH=8.3��NaHCO3��Һ��c(Na+)��c(HCO3��)��c(CO32-)��c(H2CO3)
D. pH=4��Ũ�Ⱦ�Ϊ0.1mol��L-1��CH3COOH��CH3COONa�����Һ�У�c(CH3COO��)��c(CH3COOH)=2[c(H��)��c(OH��)]=![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
A. 2L 0.5mol/L��������Һ�к��е�H+������Ϊ2NA
B. �����£�1L pH=13��NaOH��Һ�У���ˮ�����OH-������ĿΪ0.1NA
C. ��⾫��ͭ�Ĺ����У�ÿת��NA������ʱ�������ܽ�ͭ������Ϊ32g
D. ij�ܱ�������ʢ��0.1mol N2��0.3mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿС��0.6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
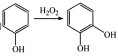
����Ŀ���佺��һ����Ȼ����ҩ����Ҫ���Գɷ�Ϊ�����ᱽ����(J)���ϳɻ�����I��·�����£�
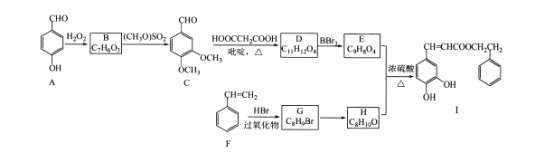
��֪����
��RCHO+HOOCCH2COOH![]() RCH=CHCOOH
RCH=CHCOOH
�۵��ǻ���˫��̼ԭ������ʱ������ת����RCH-CHOH=RCHCHO
��ش��������⣺
(l)������F��������____;B-C�ķ�Ӧ������____��
(2)������E�к��������ŵ�������____��G-H�ķ�Ӧ�����Լ��������ֱ���____��____��
(3)д��������C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ____��
(4)������W��E��Ϊͬ���칹�壬���������������������Ŀ��ȫ��ͬ���ұ�������3��ȡ��������W���ܵĽṹ��____��(������˳���칹)�����к˴Ź���������ʾ��6�ֲ�ͬ��ѧ�������⣬�������Ϊ2:2:1��1:1:1��д������Ҫ���W�Ľṹ��ʽ____��
(5)���������ϳ�·�ߣ������CH3CH=CH2��HOOCCH2COOHΪԭ���Ʊ� CH3CH2CH=CHCOOH�ĺϳ�·��(���Լ���ѡ)____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com