
(9��)��������֮���ܹ�������ͼ��ʾ�Ļ�ѧ��Ӧ���Ͻ������ֽ�����ɣ�ȡC��Һ������ɫ��Ӧ�����ʻ�ɫ���ڷ�Ӧ�в�����ˮ��δ��ͼ�б��������G��H�Ļ�ѧ����ʽΪ��4Fe(OH)2+O2+2H2O=4Fe(OH)3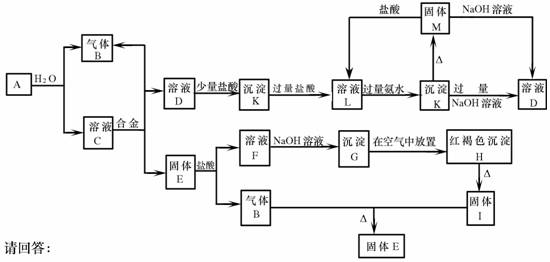
��1��д���������ʵĻ�ѧʽ��A�� D�� F��
��2��������з�Ӧ�ĵ���ת�������4Fe(OH)2 + O2 + 2H2O === 4Fe(OH)3
��3��д�����з�Ӧ�����ӷ���ʽ��
A��B�� ��
K��D�� ��
��1��A��Na D��NaAlO2 F��FeCl2
��3��A��B�� 2Na+2H2O=2Na++2OH-+H2��
K��D�� Al(OH)3+OH-=AlO2-+2H2O
��������������������⡰G��H�Ļ�ѧ����ʽΪ��4Fe(OH)2+O2+2H2O=4Fe(OH)3������֪���Ͻ�����FΪ�Ȼ��������ɡ�C��Һ������ɫ��Ӧ�����ʻ�ɫ������֪��AΪ�ƣ���ת��ͼD®K®L®D®Kת�����̣�����֪:DΪƫ�����ƣ�KΪ����������LΪ�Ȼ�����
��1��A��Na D��NaAlO2 F��FeCl2
��2��Fe���ϼ���+2����+3��O��0�۽���-2��,ת�Ƶĵ�����Ϊ4e��
��3��A��BΪ����ˮ��Ӧ�� 2Na+2H2O=2Na++2OH-+H2��
K��D�����������������Ʒ�Ӧ�� Al(OH)3+OH-=AlO2-+2H2O
���㣺�ơ������������Ļ�ѧ����
��������������������ʵĻ�ѧ���ʺ�������Ӧ�������ƶ���Ĺؼ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| 5 |
| 2 |
| 5 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
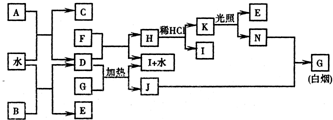
(9��) A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
(1)A��B��C��D��E��F��������������ͬһ��Ԫ����Ԫ�����ڱ���λ��________��
(2)д��C��H���ʵĻ�ѧʽ��C________��H________��
(3)д����Ӧ�٢ߵĻ�ѧ����ʽ��
��Ӧ�٣�______________________________________________________________��
��Ӧ�ߣ�______________________________________________________________��
(4)��Ӧ�����е�������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ��һ12���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
(9��)��������֮���ܹ�������ͼ��ʾ�Ļ�ѧ��Ӧ���Ͻ������ֽ�����ɣ�ȡC��Һ������ɫ��Ӧ�����ʻ�ɫ���ڷ�Ӧ�в�����ˮ��δ��ͼ�б��������G��H�Ļ�ѧ����ʽΪ��4Fe(OH)2+O2+2H2O=4Fe(OH)3
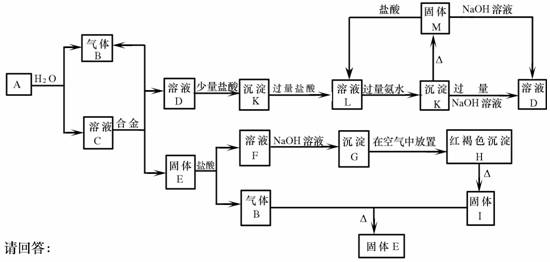
��1��д���������ʵĻ�ѧʽ��A�� D�� F��
��2��������з�Ӧ�ĵ���ת�������4Fe(OH)2 + O2 + 2H2O === 4Fe(OH)3
��3��д�����з�Ӧ�����ӷ���ʽ��
A��B�� ��
K��D�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com