
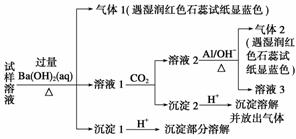
ÓŠŅ»Ęæ³ĪĒåČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬ÓŠNH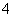 ”¢K£«”¢Na£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢Cl£”¢I£”¢NO
”¢K£«”¢Na£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢Cl£”¢I£”¢NO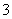 ”¢CO
ӢCO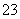 ӢSO
”¢SO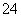 ÖŠµÄ¼øÖÖ”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃ锣
ÖŠµÄ¼øÖÖ”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃ锣
(1)ĢīŠ“±ķÖŠæÕ°×£ŗ
| ŹµŃé²½Öč | æĻ¶Ø²»“ęŌŚµÄĄė×Ó |
| ¢ŁÓĆpHŹŌÖ½¼ģŃ飬ČÜŅŗ³ŹĒæĖįŠŌ | |
| ¢ŚČ”³ö²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮæCCl4¼°ŹżµĪŠĀÖʵÄĀČĖ®£¬¾Õńµ“”¢¾²ÖĆŗóCCl4²ć³Ź×ĻŗģÉ« | |
| ¢ŪĮķČ”²æ·ÖČÜŅŗ£¬ĻņĘäÖŠ¼ÓČėNaOHČÜŅŗ£¬Ź¹ČÜŅŗ“ÓĖįŠŌÖš½„±äĪŖ¼īŠŌ£¬ŌŚµĪ¼Ó¹ż³ĢÖŠ¼°µĪ¼ÓĶź±Ļŗ󣬾łĪŽ³Įµķ²śÉś | |
| ¢ÜČ”¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ¼ÓČČ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶ | |
| ¢ŻĮķČ”¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ£¬ĻņĘäÖŠ¼ÓČėNa2CO3ČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś |
(2)øł¾ŻŅŌÉĻŹĀŹµ£¬øĆČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒ________”£
(3)Š“³öŹµŃé¢Ś”¢¢Ü”¢¢ŻÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ŹµŃé¢Ś________________________________________________________________________£»
ŹµŃé¢Ü________________________________________________________________________£»
ŹµŃé¢Ż________________________________________________________________________”£
“š°ø””(1)¢ŁCO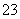 ””¢ŚFe3£«”¢NO
””¢ŚFe3£«”¢NO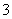 ””¢ŪMg2£«”¢Al3£«
””¢ŪMg2£«”¢Al3£«
¢ÜĪŽ””¢ŻSO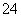
(2)I£”¢NH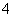 ”¢Ba2£«
”¢Ba2£«
(3)Cl2£«2I£===2Cl££«I2
NH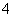 £«OH£
£«OH£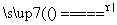 NH3”ü£«H2O
NH3”ü£«H2O
Ba2£«£«CO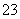 ===BaCO3”ż
===BaCO3”ż
½āĪö””¢ŁČÜŅŗ³ŹĒæĖįŠŌæĻ¶ØÓŠH£«£¬¹ŹæĻ¶Ø²»ŗ¬ÓŠCO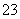 £»¢Ś¼ÓČėÉŁĮæCCl4¼°ŹżµĪŠĀÖĘĀČĖ®£¬¾Õńµ“”¢¾²ÖĆŗóCCl4²ć³Ź×ĻŗģÉ«£¬Ņ»¶Øŗ¬ÓŠI££¬¹ŹæĻ¶Ø²»ŗ¬ÓŠFe3£«”¢NO
£»¢Ś¼ÓČėÉŁĮæCCl4¼°ŹżµĪŠĀÖĘĀČĖ®£¬¾Õńµ“”¢¾²ÖĆŗóCCl4²ć³Ź×ĻŗģÉ«£¬Ņ»¶Øŗ¬ÓŠI££¬¹ŹæĻ¶Ø²»ŗ¬ÓŠFe3£«”¢NO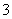 £»¢ŪŌŚµĪČėNaOHČÜŅŗ¹ż³ĢÖŠ¼°µĪ¼ÓĶź±Ļŗ󣬾łĪŽ³Įµķ²śÉś£¬¹ŹæĻ¶Ø²»ŗ¬ÓŠMg2£«”¢Al3£«£»¢Ü½«¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ¼ÓČČ£¬ÓŠÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢå·Å³ö£¬¹ŹæĻ¶Øŗ¬ÓŠNH
£»¢ŪŌŚµĪČėNaOHČÜŅŗ¹ż³ĢÖŠ¼°µĪ¼ÓĶź±Ļŗ󣬾łĪŽ³Įµķ²śÉś£¬¹ŹæĻ¶Ø²»ŗ¬ÓŠMg2£«”¢Al3£«£»¢Ü½«¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗ¼ÓČČ£¬ÓŠÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢå·Å³ö£¬¹ŹæĻ¶Øŗ¬ÓŠNH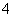 £»¢ŻĻņ¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗÖŠ¼ÓĢ¼ĖįÄĘČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬æĻ¶Øŗ¬ÓŠBa2£«£¬ŌņæĻ¶Ø²»ŗ¬ÓŠSO
£»¢ŻĻņ¢ŪÖŠµÄ²æ·Ö¼īŠŌČÜŅŗÖŠ¼ÓĢ¼ĖįÄĘČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś£¬æĻ¶Øŗ¬ÓŠBa2£«£¬ŌņæĻ¶Ø²»ŗ¬ÓŠSO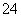 ”£×ŪÉĻĖłŹö£¬ŌČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒBa2£«”¢I£”¢NH
”£×ŪÉĻĖłŹö£¬ŌČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒBa2£«”¢I£”¢NH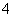 £»æĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒCO
£»æĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒCO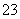 ”¢Fe3£«”¢NO
”¢Fe3£«”¢NO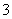 ”¢Mg2£«”¢Al3£«”¢SO
”¢Mg2£«”¢Al3£«”¢SO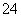 £»²»ÄÜČ·¶ØŹĒ·ń“ęŌŚµÄĄė×ÓŹĒK£«”¢Na£«”¢Cl£”£
£»²»ÄÜČ·¶ØŹĒ·ń“ęŌŚµÄĄė×ÓŹĒK£«”¢Na£«”¢Cl£”£


 ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×鏿¾ŻÖŠ£¬Ē°ÕßøÕŗĆŹĒŗóÕßĮ½±¶µÄŹĒ (”””” )
A£®2 molĖ®µÄĦ¶ūÖŹĮæŗĶ1 molĖ®µÄĦ¶ūÖŹĮæ
B£®200 mL 1 mol/LĀČ»ÆøĘČÜŅŗÖŠc(Cl£)ŗĶ100 mL 2 mol/LĀČ»Æ¼ŲČÜŅŗÖŠc(Cl£)
C£®64 g¶žŃõ»ÆĮņÖŠŃõŌ×ÓŹżŗĶ±ź×¼×“æöĻĀ22.4 LŅ»Ńõ»ÆĢ¼ÖŠŃõŌ×ÓŹż
D£®20%NaOHČÜŅŗÖŠNaOHµÄĪļÖŹµÄĮæÅضČŗĶ10%NaOHČÜŅŗÖŠNaOHµÄĪļÖŹµÄĮæÅضČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķź³ÉĻĀĮŠĄė×Ó·½³ĢŹ½£ŗ
(1)ÄĘÓėĖ®·“Ó¦£ŗ_______________________________________________________________”£
(2)ĀČĘųĶØČėĖ®ÖŠ£ŗ_____________________________________________________________”£
(3)ĻņĒāŃõ»ÆÄĘČÜŅŗÖŠĶØČėÉŁĮæCO2£ŗ
________________________________________________________________________ӣ
(4)Ģ¼ĖįøĘÖŠµĪČė“×ĖįČÜŅŗ£ŗ
________________________________________________________________________ӣ
(5)ĀĮʬĶ¶ČėĒāŃõ»ÆÄĘČÜŅŗ£ŗ
________________________________________________________________________ӣ
(6)ĀČ»ÆĀĮČÜŅŗÖŠ¼Ó×ćĮæĢ¼ĖįĒāÄĘČÜŅŗ£ŗ
________________________________________________________________________ӣ
(7)FeCl3ČÜŅŗÓėCu·“Ó¦£ŗ
________________________________________________________________________ӣ
(8)ĖįŠŌĮņĖįŃĒĢśČÜŅŗÖŠ¼ÓČė¹żŃõ»ÆĒāČÜŅŗ£ŗ
________________________________________________________________________ӣ
(9)ŹµŃéŹŅÓĆMnO2ÓėÅØŃĪĖįÖĘČ”Cl2£ŗ
________________________________________________________________________ӣ
(10)NO2ČÜÓŚĖ®£ŗ_____________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ(Ė«Ń”)(””””)
A£®ĻņAl2(SO4)3ČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£ŗAl3£«£«3NH3·H2O===Al(OH)3”ż£«3NH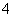
B£®½«Fe(OH)2ČÜÓŚ¹żĮæµÄĻ”ĻõĖį£ŗFe(OH)2£«2H£«===Fe2£«£«2H2O
C£®ÓĆNaClOČÜŅŗĪüŹÕ¹żĮæµÄSO2£ŗClO££«SO2£«H2O===SO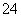 £«Cl££«2H£«
£«Cl££«2H£«
D£®Fe(OH)3ČÜÓŚĒāµāĖį£ŗFe(OH)3£«3H£«===Fe3£«£«3H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Īķö²ŃĻÖŲÓ°ĻģČĖĆĒµÄÉś»īÓė½”æµ”£Ä³µŲĒųµÄĪķö²ÖŠæÉÄÜŗ¬ÓŠČēĻĀæÉČÜŠŌĪŽ»śĄė×Ó£ŗNa£«”¢NH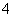 ”¢Mg2£«”¢Al3£«”¢SO
”¢Mg2£«”¢Al3£«”¢SO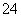 ”¢NO
”¢NO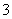 ”¢Cl£ ”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
”¢Cl£ ”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
ŅŃÖŖ£ŗ3NO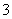 £« 8Al£«5OH££«2H2O
£« 8Al£«5OH££«2H2O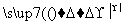 3NH3”ü£«8AlO
3NH3”ü£«8AlO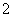
øł¾ŻŅŌÉĻµÄŹµŃé²Ł×÷ÓėĻÖĻó£¬øĆĶ¬Ń§µĆ³öµÄ½įĀŪ²»ÕżČ·µÄŹĒ(””””)
A£®ŹŌŃłÖŠæĻ¶Ø“ęŌŚNH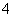 ”¢Mg2£«”¢SO
”¢Mg2£«”¢SO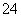 ŗĶNO
ŗĶNO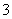
B£®ŹŌŃłÖŠŅ»¶Ø²»ŗ¬Al3£«
C£®ŹŌŃłÖŠæÉÄÜ“ęŌŚNa£«”¢Cl£
D£®øĆĪķö²ÖŠæÉÄÜ“ęŌŚNaNO3 ”¢NH4ClŗĶMgSO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ŌŚ»Æѧ·“Ó¦¹ż³ĢÖŠ£¬·¢ÉśĪļÖŹ±ä»ÆµÄĶ¬Ź±²»Ņ»¶Ø·¢ÉśÄÜĮæ±ä»Æ
B£®ĘĘ»µ·“Ó¦²śĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæ“óÓŚĘĘ»µ·“Ó¦ĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæŹ±£¬·“Ó¦ĪŖĪüČČ·“Ó¦
C£®·“Ó¦²śĪļµÄ×ÜģŹ“óÓŚ·“Ó¦ĪļµÄ×ÜģŹŹ±£¬·“Ó¦ĪüČČ£¬¦¤H>0
D£®¦¤HµÄ“óŠ”ÓėČČ»Æѧ·½³ĢŹ½µÄ¼ĘĮæĻµŹżĪŽ¹Ų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ
(1)H2(g)£«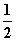 O2(g)===H2O(g) ¦¤H1£½a kJ·mol£1
O2(g)===H2O(g) ¦¤H1£½a kJ·mol£1
(2)2H2(g)£«O2(g)===2H2O(g) ¦¤H2£½b kJ·mol£1
(3)H2(g)£«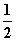 O2(g)===H2O(l) ¦¤H3£½c kJ·mol£1
O2(g)===H2O(l) ¦¤H3£½c kJ·mol£1
(4)2H2(g)£«O2(g)===2H2O(l) ¦¤H4£½d kJ·mol£1
ĻĀĮŠ¹ŲĻµŹ½ÖŠÕżČ·µÄŹĒ(””””)
A£®a<c<0 B£®b>d>0 C£®2a£½b<0 D£®2c£½d>0
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«1 mol±ł“×Ėį¼ÓČėµ½Ņ»¶ØĮæµÄÕōĮóĖ®ÖŠ×īÖÕµĆµ½1 LČÜŅŗ”£ĻĀĮŠø÷ĻīÖŠ£¬±ķÕ÷ŅŃ“ļµ½µēĄėĘ½ŗāדĢ¬µÄŹĒ(””””)
A£®“×ĖįµÄÅØ¶Č“ļµ½1 mol”¤L£1
B£®[H£«]µÄÅØ¶Č“ļµ½0.5 mol”¤L£1
C£®[CH3COOH]”¢[CH3COO£]”¢[H£«]¾łĪŖ0.5 mol”¤L£1
D£®“×Ėį·Ö×ÓµēĄė³ÉĄė×ÓµÄĖŁĀŹŗĶĄė×ÓÖŲŠĀ½įŗĻ³É·Ö×ÓµÄĖŁĀŹĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷ČÜŅŗÖŠ£¬Į£×ÓµÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ( )
A£®0.1 mol”¤L£1µÄCH3COONaČÜŅŗÖŠ£ŗc(CH3COO£)>c(Na£«)>c(H£«)>c(OH£)
B£®0.1 mol”¤L£1µÄNa2CO3ČÜŅŗÖŠ£ŗc(OH£)£½c(HCO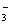 )+c(H£«)+c(H2CO3)
)+c(H£«)+c(H2CO3)
C£®0.1 mol”¤L£1µÄNH4ClČÜŅŗÖŠ£ŗC(Cl£)>C(NH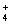 )>c(OH£)>c(H£«)
)>c(OH£)>c(H£«)
D£®c(NH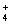 )ĻąµČµÄ(NH4)2SO4ČÜŅŗ”¢NH4HSO4ČÜŅŗ”¢(NH4)2CO3ČÜŅŗ”¢NH4ClČÜŅŗ£ŗ
)ĻąµČµÄ(NH4)2SO4ČÜŅŗ”¢NH4HSO4ČÜŅŗ”¢(NH4)2CO3ČÜŅŗ”¢NH4ClČÜŅŗ£ŗ
c< c<c(NH4HSO4)<c(NH4Cl)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com